Synthesis, characterization and immobilization of a new cobalt(II) complex on modified magnetic nanoparticles as catalyst for epoxidation of alkenes and oxidation of activated alkanes†
Zeinab Asgharpoura,
Faezeh Farzaneh*a and
Alireza Abbasib
aDepartment of Chemistry, Faculty of Physics & Chemistry, Alzahra University, P.O. Box 1993891176, Vanak, Tehran, Iran. E-mail: faezeh_farzaneh@yahoo.com; Farzaneh@alzahra.ac.ir; Fax: +98-21-88041344; Tel: +98-21-88258977
bSchool of Chemistry, College of Science, University of Tehran, P.O. Box 14155 6455, Tehran, Iran
First published on 3rd October 2016
Abstract
The [Co(2-hydroxy-1-naphthaldehyde)2(DMF)2] complex 1 was prepared and characterized by X-ray crystallography, successfully supported on modified Fe3O4 nanoparticles using tetraethylorthosilicate (TEOS) and (3-aminopropyl)trimethoxysilane (APTMS) and designated as Fe3O4@SiO2@APTMS@complex nanocatalyst. FT-IR, elemental analysis, XRD, VSM and TEM studies have been used to characterize the nanocatalyst. The catalytic activity of Fe3O4@SiO2@APTMS@complex was evaluated by the epoxidation of norbornene, cyclooctene, styrene, α-methyl styrene, trans-stilbene and limonene, with 50–100% conversions and 83–100% selectivities. Furthermore, it was found that Fe3O4@SiO2@APTMS@complex successfully catalyzes the oxidation of activated secondary alkanes such as fluorene, adamantane, diphenyl methane, and ethylbenzene with 40–100% conversions and 80–100% selectivities toward the corresponding products. Based on the obtained results, the heterogeneity and reusability of the catalyst seems promising.
Introduction
The catalytic oxidation of alkanes and alkenes has been a subject of growing interest in the production of chemicals and fine chemicals. New active and selective epoxidation catalysts have been developed due to the key role of epoxides as starting materials for a wide variety of products. On the other hand, whereas alkanes are the most abundant and least expensive chemicals for the production of valuable products, methods of selective oxidations are either rare or inefficient.1–8 Transition metal complexes have successfully been used to catalyze the oxidation of hydrocarbons under moderate reaction conditions,2,9,10 amongst which different cobalt complexes are of particular significance.11 Interestingly, cobalt compounds not only are used as active catalysts for oxidation and epoxidation of hydrocarbons with low cost and less environmentally damaging, but also are used by nature as active catalysts in a variety of metalloenzymes.12 Cobalt salts and cobalt containing coordination complexes are well-known catalysts for the selective oxidation of alkanes and selective epoxidation of alkenes and quite a number of studies have been conducted. This includes cobalt(III) acetylacetonate (acac)/O2,13 cobalt(II)–salen complexes/O2/isobutyrylaldehyde,14 cobalt(II) calix[4] pyrrole complexes/2-ethylbutyraldehyde/O2,15 Schiff base cobalt(II) complexes/aliphatic aldehydes or β-ketoesters,16 polymer supported cobalt(II) complex/O2, H2O2,17 (N-hydroxyl-phthalimide)/Co(OAc)2/O2 (ref. 18) and cobalt(II) phthalocyanine.19,20On the other hand, the coordination chemistry of ligands derived from catechols and o-benzoquinones has been developed, primarily, over the past twenty five years with interesting and surprising results.21–23 Initial interest in these complexes was associated with the redox activity of the quinone ligands and the potential for forming compounds that may exist in a number of electronic states due to the combined electrochemical activity of the cobalt ion and one or more quinone ligands.23
Homogeneous catalysis has been practiced for a very long time in large-scale oxidation reactions. However, these homogeneous catalysts face the problem of separation from the reaction mixture and reuse; they very often decompose during the catalytic reactions. A homogeneous complex catalyst can be recovered and reused if it is heterogenized on an insoluble support.24,25 The dispersion and attachment of catalytically-active metallic nanoparticles or organometallic complexes onto polymeric or inorganic solid support materials such as silica,26 inorganic layers,27 microporous and mesoporous such as zeolites, MCM-41 and SBA15,28–30 and magnetic nanoparticles31,32 via non-covalent or covalent interactions can generally produce effective heterogeneous catalysts for numerous reactions including oxidation of alkanes and alkenes.33–36 In this study, a new cobalt complex with 2-hydroxynaphthaldehyde was prepared and characterized followed by immobilization on modified iron oxide nanoparticles as heterogeneous catalyst for oxidation reactions of alkanes and alkenes.
Results and discussion
[Co(2-hydroxy-1-naphthaldehyde)2(DMF)2] complex 1 was prepared from reaction of 2-hydroxy-1-naphthaldehyde and CoCl2·6H2O in the presence of melamine and sodium acetate in ethanol and water under reflux condition.Description of crystal structure of complex
The crystal structure of complex 1 with atom-numbering is shown in Fig. 1. Basic crystal data, diffraction description and details of the structure refinement are given in Table S1.† Selected bond distances and angels are presented in Table 1. | ||
| Fig. 1 An ORTEP view of complex 1 drawn at 50% ellipsoid probability levels with the crystallographic numbering. | ||
| a Symmetry code: (i) −x + 1, −y + 1, −z. | |
|---|---|
| Bond lengths (Å) | |
| Co(1)–O(2)i | 1.989 (2) |
| Co(1)–O(2) | 1.989 (2) |
| Co(1)–O(3)i | 2.059 (2) |
| Co(1)–O(3) | 2.059 (2) |
| Co(1)–O(1)i | 2.191 (2) |
| Co(1)–O(1) | 2.191 (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Bond angles (°) | |
| O(2)i–Co(1)–O(2) | 180.0 |
| O(2)i–Co(1)–O(3)i | 87.71 (8) |
| O(2)–Co(1)–O(3)i | 92.29 (8) |
| O(2)i–Co(1)–O(3) | 92.29 (8) |
| O(2)–Co(1)–O(3) | 87.71 (8) |
| O(3)i–Co(1)–O(3) | 180.0 |
| O(2)i–Co(1)–O(1)i | 88.97 (9) |
| O(2)–Co(1)–O(1)i | 91.03 (9) |
| O(2)–Co(1)–O(1) | 88.97 (9) |
| O(3)i–Co(1)–O(1) | 90.02 (9) |
| O(3)–Co(1)–O(1) | 89.71 (9) |
| O(1)i–Co(1)–O(1) | 180.000 (1) |
The crystal structure of 1 was satisfactorily described in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with Co(II) ion in a distorted octahedral 6-coordinated geometry. Bond angles in the octahedron vary from 88.97(9)° to 91.03(9)° for cis and 180° for trans-substituted, indicating the small distortion degree in the octahedron.
with Co(II) ion in a distorted octahedral 6-coordinated geometry. Bond angles in the octahedron vary from 88.97(9)° to 91.03(9)° for cis and 180° for trans-substituted, indicating the small distortion degree in the octahedron.
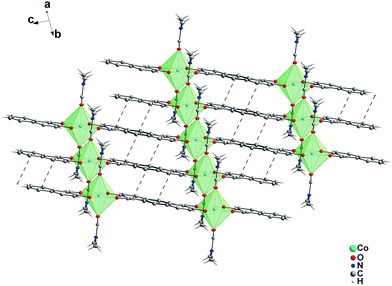
The asymmetric unit of 1 is composed of one DMF (dimethylformamide) molecule and one 2-hydroxy-1-naphthaldehyde anion. Two 2-hydroxy-1-naphthaldehyde ligands coordinate to Co(II) as bidentate [O2, O3], chelating ligand via the deprotonated carboxylic acid and phenoxy oxygen atoms, defining the equatorial coordination plane. There is a distinct trend in Co–O bond lengths in the complex with Co1–O2 (carboxyl) < Co1–O3 (phenoxyl) < Co1–O1 (dimethylformamide). The complexes are held together through hydrogen bonds (C3–H3A⋯O3) (C3–H3A: 0.9600 (Å), H3A⋯O3: 2.4800(Å), C3⋯O3: 3.353(4) (Å), C3–H3A⋯O3: 151.00°) making a chain along [100] (Fig. 2). The neighboring chains are then connected by pi⋯pi interactions (Cg(3)⋯Cg(4), 4.161 Å, where Cg(3): C4/C5/C7/C8/C9/C10 and Cg(4): C7/C8/C11/C12/C13/C14) stabilizing 3D framework (Fig. 3).
The FT-IR spectra of free 2-hydroxy-1-naphthaldehyde as ligand and [Co(2-hydroxy-1-naphthaldehyde)2(DMF)2] complex 1 are shown in Fig. 4a and b, respectively. The bands appearing at 3100, 2855, 1647, 1180 and 770 cm−1 are due to the phenolic OH, C–H, aldehyde C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phenolic C–O stretching and O–H bending, respectively (Fig. 4a). The absence of absorption bands at 3100 and 770 cm−1 due to the stretching and bending of phenolic OH in the FT-IR spectrum of complex 1 confirms the bond formation between cobalt ion and phenolic oxygen via deprotonation. Moreover, the 10 cm−1 increase in the wave number of band appearing at 1119 cm−1 is due to the phenolic C–O absorption which also confirms the CO coordination to the metal.37 The red shift observed in C
O, phenolic C–O stretching and O–H bending, respectively (Fig. 4a). The absence of absorption bands at 3100 and 770 cm−1 due to the stretching and bending of phenolic OH in the FT-IR spectrum of complex 1 confirms the bond formation between cobalt ion and phenolic oxygen via deprotonation. Moreover, the 10 cm−1 increase in the wave number of band appearing at 1119 cm−1 is due to the phenolic C–O absorption which also confirms the CO coordination to the metal.37 The red shift observed in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O wave number appearing at 1641 cm−1 indicates the oxygen atom coordination to the cobalt ion with no need to enolization.38
O wave number appearing at 1641 cm−1 indicates the oxygen atom coordination to the cobalt ion with no need to enolization.38
 | ||
| Fig. 4 FT-IR spectra of (a) 2-hydroxy-1-naphthaldehyde and (b) [Co(2-hydroxy-1-naphthaldehyde)2(DMF)2] complex 1. | ||
The electronic absorption spectrum of complex 1 is shown in Fig. S1.† The cobalt(II) complex 1 in DMF exhibits three bands at 266, 313 and 369 nm, and a broad band with low absorptivity at 416 nm. The first band observed at 266 nm can be assigned to the aromatic ring transition π → π*. The other bands displaying at 313 and 369 are due to n → π* type electronic transitions. Broad band with low absorptivity centred at 416 nm can be assigned to a d–d transition from the 3d cobalt(II) metal center.39
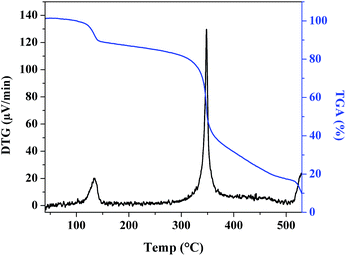
To evaluate the thermal stability of complex 1, thermal decomposition was studied (Fig. 5). The TGA curve shows two step weight loss at 133 and 348 °C together with exothermic peaks in the DTG curve due to the decomposition of the dimethylformamide and organic ligand (obs.: 89.5%, calc.: 89.2%) with the formation of cobalt oxide.
Characterization of Fe3O4@SiO2@APTMS@complex 1
Fe3O4 nanoparticles (MNPs) were prepared by precipitation of iron(II) and (III) ions in basic solution under nitrogen atmosphere.40 Subsequent coating with silica to forming Fe3O4@SiO2 core–shell particles followed by modification with aminopropyl-trimethoxysilane (APTMS), afforded MNPs designated as Fe3O4@SiO2@APTMS. Finally, immobilization of complex 1 within Fe3O4@SiO2@APTMS resulted in the formation of the desired product designated as Fe3O4@SiO2@APTMS@complex 1, perhaps by the exchange of the immobilized amine ligand with coordinated DMF (Scheme 1).As shown in Fig. 6a, the broad band with relatively low intensity centered at 3400 and 1600 cm−1 should be attributed either to the surface Fe–OH groups, or the stretching and bending vibrations of the adsorbed water. The stretching vibration of Fe–O also appears at 555 cm−1. The band appearing at 1091 cm−1 due to Si–O stretching demonstrates the presence of SiO2 components (Fig. 6b).40–42 After functionalization of silica coated magnetic nanoparticles with APTMS, new bands displaying at 2870–2920 cm−1 are due to the N–H and C–H stretching vibrations.43 The FT-IR spectrum of catalyst 1 is shown in Fig. 6d. Upon immobilization of cobalt complex on the modified nanoparticles, the appearance of two peaks at 1647 and 1180 cm−1 due to the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and ν(C–O), respectively confirms the occurrence of immobilization.
O) and ν(C–O), respectively confirms the occurrence of immobilization.
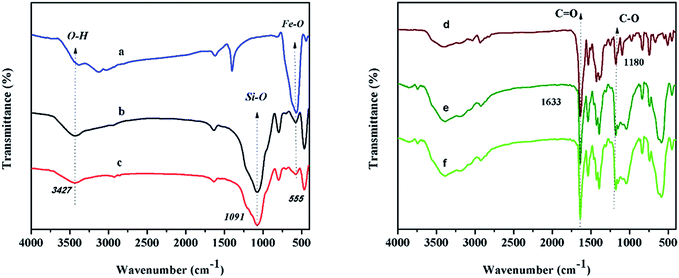 | ||
| Fig. 6 The FTIR spectra of (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2@APTMS, (d) complex 1, (e) Fe3O4@SiO2@APTMS@complex 1 before using and (f) after using as catalyst. | ||
To study the magnetic properties of magnetite nanoparticles, the hysteresis loops of magnetite and functionalized magnetite nanoparticles at room temperature were investigated by using vibrating sample magnetometry (VSM). Magnetization curves of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2@APTMS, Fe3O4@SiO2@APTMS@complex 1 before and after using as catalyst are shown in Fig. 7a–c, respectively. The magnetization curve of Fe3O4 exhibited superparamagnetic property with saturation magnetization of about 61 emu g−1. The silica coated Fe3O4 magnetite nanoparticles also showed superparamagnetic behavior with decreased saturation magnetization of about 57 emu g−1. After coating of Fe3O4@SiO2 nanoparticles with aminosilane, the superparamagnetic behavior of Fe3O4@SiO2@APTMS was found to be about 56 emu g−1. Immobilization complex 1 within the Fe3O4@SiO2@APTMS surface exhibited superparamagnetic property decrease to saturation magnetization of about 51 emu g−1. Recall that the superparamagnetic property of Fe3O4@SiO2@APTMS@complex 1 prevents aggregation and enables it to disperse rapidly when the magnetic field is removed.40,42,44
 | ||
| Fig. 7 Magnetization curves of (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2@APTMS, (d) Fe3O4@SiO2@APTMS@complex 1 before and (e) after using as catalyst. | ||
The X-ray diffraction pattern of (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2@APTMS, (d) complex 1 (e) Fe3O4@SiO2@APTMS@complex 1 before and (f) after using as catalyst are shown in Fig. 8a–f, respectively. As shown in Fig. 8a, it can be seen that the obtained Fe3O4 has highly crystalline cubic spinel structure in agree with the standard Fe3O4 (cubic phase) XRD spectrum (JCPDS no. 19-0629).45,46 The similar set of characteristic peaks observed for Fe3O4@SiO2 and Fe3O4@SiO2@APTMS (Fig. 8b and c) indicate the stability of the crystalline phase of silica coating and surface functionalization. The XRD pattern of complex 1 and Fe3O4@SiO2@APTMS@complex 1 are shown in Fig. 8d and e, respectively. Observation of two additional peak at 2 theta 15 and 18 for Fe3O4@SiO2@APTMS@complex 1 should be attributed to the immobilization of complex 1 on modified iron oxide magnetic nanoparticles.
 | ||
| Fig. 8 XRD pattern for (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2@APTMS (d) complex 1 (e) Fe3O4@SiO2@APTMS@complex 1 before using and (f) after using as catalyst. | ||
The EDX of Fe3O4@SiO2@APTMS@complex 1 confirmed the presence of cobalt complex on modified iron nanomagnet (Fig. 9).
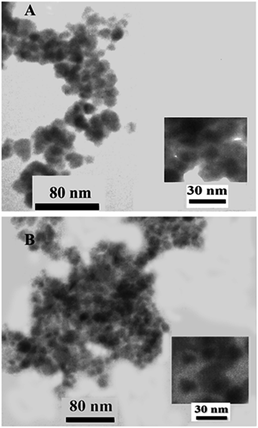
The TEM image of Fe3O4@SiO2@APTMS@complex 1 presented in Fig. 10A and B before and after using it as catalyst show the core and shell of the prepared nanoparticles with the average particle size of 10 nm.
Catalytic studies
Reaction of norbornene epoxidation with TBHP as oxidant was selected as model to investigate the catalytic activity of Fe3O4@SiO2@APTMS@complex 1 (designated as catalyst 2) and other reaction parameters such as catalyst amount, reaction time and solvent. The results are presented in Fig. 11–13. Excellent reaction yield was obtained using 25 mg of catalyst in refluxing CH3CN within 3 h. As seen in Fig. 11, increasing the amount of catalyst from 5 to 25 mg increases the conversion from 33 to 100% with 100% selectivity. Therefore, all epoxidation reactions were carried out using 25 mg of catalyst. Similarly, increasing the reaction time from 0.5 to 3 h using 25 mg of catalyst was found to increase the norbornene conversion from 17 to 100% with 100% selectivity (Fig. 12).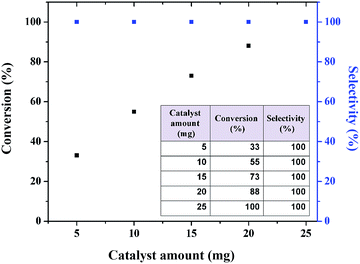 | ||
| Fig. 11 Conversion and selectivity of norbornene with different amounts of Fe3O4@SiO2@APTMS@complex 1 as catalyst 2. | ||
 | ||
| Fig. 12 Conversion and selectivity of norbornene at different times in the presence of the Fe3O4@SiO2@APTMS@complex 1 as catalyst 2. | ||
 | ||
| Fig. 13 The effect of solvent on the conversion and selectivity of norbornene using Fe3O4@SiO2@APTMS@complex 1 as catalyst 2. Ethylbenzene were investigated under optimized reaction conditions. | ||
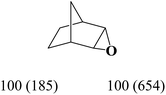
The nature of solvent has an important role on the conversion and product selectivity. In this study, both protic and aprotic solvents were used to investigate the solvent effect on the norbornene epoxidation. As seen in Fig. 13, protic solvents such as methanol and ethanol were not suitable for norbornene epoxidation. These results are similar to those previously reported for alkene epoxidation studies.47–49 On the other hand, high conversion and selectivity toward epoxide was achieved in aprotic solvents in the order of n-hexane < CHCl3 < CH2Cl2 < CH3CN. The observed trend which is consistent with increasing order of dielectric constants clearly indicates the increasing solvent polarity role toward high conversion (100%) and high epoxide selectivity (100%) in norbornene epoxidation. When epoxidation of norbornene was carried out in the presence of both complex 1 (catalyst 1) and Fe3O4@SiO2@APTMS@complex 1 as catalyst 2, 100% conversion and selectivity was observed within 6 h in CH3CN. Inspired by these results, other alkenes such as cyclooctene, styrene, α-methyl styrene, trans-stilbene and cyclohexene and activated secondary alkanes including fluorene, adamantane, diphenylmethane and (Tables 2 and 3). As seen in Table 2, the reaction results can be categorized in three trends. Whereas norbornene or cyclooctene were selectively converted to the corresponding epoxides (entries 1 and 2, Table 2), epoxidation of α-methyl styrene, styrene and trans-stilbene proceeded to the corresponding epoxides as well as another by-product in each case (entries 3 to 5, Table 2). On the other hand, cyclohexene afforded cyclohexene epoxide plus 2-cyclohexene-1-ol and 2-cyclohexene-1-one (entry 5, Table 2). Interestingly, fluorene, adamantane, diphenylmethane and ethylbenzene were oxidized to the corresponding ketones with 40–100% conversions and 100% selectivities (Table 3).
| Entry | Substrate conversiona (%) | Epoxide selectivityb (%) (TON) | ||
|---|---|---|---|---|
| Catalyst 1 | Catalyst 2 | Catalyst 1 | Catalyst 2 | |
| a Reaction conditions: catalyst: 25 mg, substrate: alkenes (10 mmol), (trans-stilbene, 1 mmol), alkanes (1 mmol) (cyclooctane, 10 mmol), TBHP (14 mmol), solvent (acetonitrile, 5 mL), conditions (reflux at 80 °C), time (1: 6 h, 2, 3 h).b TON is the mmol of product to mmol of vanadium present in catalyst.c Acetophenone was identified as byproduct.d Benzaldehyde (10%) and benzoic acid (15%) were identified as byproducts.e Benzaldehyde (7%) and benzoic acid (13%) were identified as byproducts.f Benzaldehyde (10%) and benzoic acid (20%) were identified as byproducts.g Benzaldehyde (3%) was identified as byproduct.h 2-Cyclohexene-1-ol (42%) and 2-cyclohexene-1-one (23%) were identified as byproducts.i 2-Cyclohexene-1-ol (35%) and 2-cyclohexene-1-one (12%) were identified as byproducts. | ||||
| 1 | 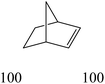 |
 |
||
| 2 |  |
 |
||
| 3 | 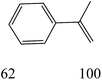 |
 |
||
| 4 | 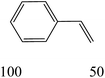 |
 |
||
| 5 | 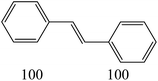 |
 |
||
| 6 | 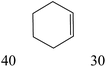 |
 |
||
| Entry | Substrate conversiona (%) | Product selectivity (%) | ||
|---|---|---|---|---|
| Catalyst 1 | Catalyst 2 | Epoxideb (TON) | ||
| Catalyst 1 | Catalyst 2 | |||
| a Reaction conditions: catalyst, 25 mg, substrate (alkanes, 1 mmol), TBHP (1.4 mmol), solvent (acetonitrile, 5 mL, reflux at 80 °C), time (1: 6 h, 2, 3 h).b TON is the mmol of product to mmol of vanadium present in catalyst.c Diphenyl methanol was identified as byproduct. | ||||
| 1 |  |
 |
||
| 2 |  |
 |
||
| 3 |  |
 |
||
| 4 |  |
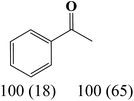 |
||
To cast light onto the reaction mechanism,50–52 the norbornene epoxidation and fluorene or adamantane oxidation reactions were carried out in the presence of diphenylamine as a radical scavenger. Observation of suppression of the reaction yields clearly revealed that these processes have proceeded via a radical mechanism.53–55 Based on the operation of a radical mechanism, one may rationalize the reaction mechanism (Scheme 2). It seems likely that reaction between catalyst and TBHP initially affords a CoIII–peroxy compound. Subsequently, t-BuOO radical is released from CoIII–peroxy adduct and regenerating the catalyst (Scheme 2a).56 In the next step, t-BuOO radical either is added to the alkene double bond and forming t-butoxperoxy I (path 1, Scheme 2a) or abstract an active H from allylic C–H bond and generating cycloalkenyl radical I′ intermediate (path 1′, Scheme 2a). Whereas elimination of t-BuOH from I affords the epoxide product (path 2, Scheme 2a), reaction of radical I′ with molecular O2 gives rise to cycloalkenylperoxide radical II′ (paths 2′, Scheme 2a), which subsequently generates 2-cycloalkenyl-1-ol and 2-cycloalkenyl-1-one via a disproportionation reaction (paths 3′, Scheme 2a). Since aqueous solution of TBHP was used as oxidant, partial hydrolysis of α-methyl styrene, styrene and trans-stilbene epoxides to the relevant 1,2-diols perhaps catalyzed by some acidic free silanol groups present on the support is inevitable (path 3, Scheme 2a).57 Subsequently, the obtained 1,2-diols undergo extremely rapid scission to acetophenone, benzaldehyde and benzaldehyde, respectively (path 4, Scheme 2a).58 Recall that norbornene and cyclooctene neither undergo hydrolysis since their 1,2-diol degradations are inefficient, nor susceptible to allylic site oxidation due to instability of the bridgehead radical intermediate of the former (Scheme 2b)59,60 and orthogonality of the C–H orbital to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the latter (Scheme 2c) respectively.61 Particularly significant is cyclohexene in which either epoxidation or oxidation take place concomitantly on C
C double bond in the latter (Scheme 2c) respectively.61 Particularly significant is cyclohexene in which either epoxidation or oxidation take place concomitantly on C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H bonds, respectively. Since radical p and C
C and C–H bonds, respectively. Since radical p and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C π-orbitals of cyclohexene are closely parallel to each other, it easily undergoes allylic-site oxidation, affording cyclohexenol and cyclohexenone (Scheme 2d).
C π-orbitals of cyclohexene are closely parallel to each other, it easily undergoes allylic-site oxidation, affording cyclohexenol and cyclohexenone (Scheme 2d).
 | ||
| Scheme 2 Proposed reaction mechanism for the epoxidation of alkene and oxidation of alkane with the catalyst. | ||
Finally, transformation mechanism of alkanes having active secondary C–H bonds such as fluorene, adamantane, diphenyl methane, and ethylbenzene to the corresponding ketones under the catalytic effect of catalysts 1 and 2 is suggested (Scheme 2e).62
The stability of the magnetically recovered Fe3O4@SiO2@APTMS@complex 1 as catalyst 2 was confirmed with spectroscopic studies and elemental analysis. The FTIR and XRD spectra of catalyst 2 before and after using as catalyst are similar. Moreover, as seen in VSM spectra after using Fe3O4@SiO2@APTMS@complex 1 as catalyst 2, the saturation magnetization was found to be 48 emu g−1. Therefore, it can be concluded that there is no significant change in its magnetic property and the magnetic core seems to be stable during epoxidation reaction.63 Cobalt and iron content of Fe3O4@SiO2@APTMS@complex 1 determined before and after using it as catalyst are 3.66%, 41.91% and 3.63%, 41.90%, respectively. Observation of no catalytic activity in the filtrate when it was subjected to the epoxidation conditions in the absence of catalyst clearly indicated that the catalyst is truly heterogeneous in nature.
The reusability of the catalyst 2 after recovery by magnetic decantation and washing with solvent was examined in the epoxidation of norbornene in another run by addition of fresh norbornene similar to the initial reaction. As indicated in Fig. 14, a decrease in conversion from 100 to 97% without any changes in selectivity was observed after five runs.
 | ||
| Fig. 14 The effect of recycling of Fe3O4@SiO2@APTMS@complex as catalyst (2) on epoxidation of norbornene. | ||
Whereas the catalytic activity of homogeneous catalyst 1 exhibits high activity in comparison to that of the heterogeneous catalyst 2 toward the formation of corresponding products (Tables 2 and 3), the latter with higher TON as indicated in these tables is considerable.
Conclusions
Co(II) complex was prepared with CoCl2·6H2O and 2-hydroxy-1-naphthaldehyde in the presence of melamine designated as [Co(2-hydroxy-1-naphthaldehyde)2(DMF)2] complex 1. The molecular structure of complex 1 was determined by single-crystal X-ray crystallography. The Co atom in complex 1 crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group and adopts a distorted octahedral 6-coordinated geometry. Two molecules of 2-hydroxy-1-naphthaldehyde coordinate as bidentate chelating ligands in equatorial position and two DMF molecules in axial positions are bound to Co(II) atom. Complex 1 immobilized on the modified iron oxide nanomagnet was designated as Fe3O4@SiO2@APTMS@complex 1. Catalytic activities of complex 1 and Fe3O4@SiO2@APTMS@complex 1 were investigated in the epoxidation of alkenes and oxidation of activated secondary alkanes with TBHP as homogenous catalyst 1 and heterogeneous catalyst 2, respectively. It was found that the selectivity and TON number of heterogeneous system is higher than that of homogenous one. Easy preparation, mild reaction conditions, high yield, ease of catalyst separation and recyclability of the solid catalyst candidates Fe3O4@SiO2@APTMS@complex 1 as a useful heterogeneous catalysis system.
space group and adopts a distorted octahedral 6-coordinated geometry. Two molecules of 2-hydroxy-1-naphthaldehyde coordinate as bidentate chelating ligands in equatorial position and two DMF molecules in axial positions are bound to Co(II) atom. Complex 1 immobilized on the modified iron oxide nanomagnet was designated as Fe3O4@SiO2@APTMS@complex 1. Catalytic activities of complex 1 and Fe3O4@SiO2@APTMS@complex 1 were investigated in the epoxidation of alkenes and oxidation of activated secondary alkanes with TBHP as homogenous catalyst 1 and heterogeneous catalyst 2, respectively. It was found that the selectivity and TON number of heterogeneous system is higher than that of homogenous one. Easy preparation, mild reaction conditions, high yield, ease of catalyst separation and recyclability of the solid catalyst candidates Fe3O4@SiO2@APTMS@complex 1 as a useful heterogeneous catalysis system.
Experimental
General remarks
All materials were commercial reagent grade without further purification. FT-IR spectra were recorded on a Bruker Tensor 27 FT-IR spectrometer using KBr pellets over the range of 4000–400 cm−1. Magnetic susceptibility measurements were carried out using a vibrating sample magnetometer (VSM) (BHV-55, Riken, Japan) in the magnetic field range of −8000 to 8000 Oe at room temperature. Cobalt and iron were determined by atomic absorption on a Chermo double beam instrument. Transmission electron microscopy (TEM) images were taken by Zeiss-EM10C-100 kV. Epoxidation products were analyzed by GC and GC-Mass using Agilent 6890 series with a FID detector, HP-5, 5% phenylmethylsiloxane capillary and Agilent 5973 network, mass selective detector, HP-5 MS 6989 network GC.X-ray crystallography
Crystallographic data were collected on a MAR345 dtb diffractometer equipped with image plate detector using Mo-Kα radiation (0.71073 Å). The structure was solved by direct methods using SHELXS-97 and refined using the full-matrix least-squares method on F2, SHELXL-97.64 All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were added at ideal positions and refined using a riding model. Crystallographic data, details of collected data and structure refinement are listed in Table S1.† Selected bond lengths and angles are shown in Table 1.Preparation of [Co(2-hydroxy-1-naphthaldehyde)2(DMF)2] complex (1)
[Co(2-hydroxy-1-naphthaldehyde)2(DMF)2] complex (1) was prepared from inducing 2-hydroxy-1-naphthaldehyde (1 mmol, 172 mg in 2 mL EtOH) into 1,3,5-triazine-2,4,6-triamine (melamine) (1 mmol, 126 mg in 2 mL H2O) in the presence of sodium acetate (2 mmol, 164 mg in 4 mL H2O) and heating at reflux condition. After appearing a clear yellow solution, CoCl2·6H2O (1 mmol, 238 mg in 4 mL EtOH) was added and the solution was heated at reflux for 5 h. The yellow resultant solid was filtered, washed with water, ethanol and diethyl ether and dried in air at room temperature. X-ray quality crystals of the complex 1 was obtained by slow evaporation of saturated resultant solid solution in dimethylformamide (82% yield). Decomp. point 275 °C, anal. calc. for C28H28CoN2O6 (M = 547.45 g mol−1): C, 61.43; H, 5.16; Co, 10.76; N, 5.12. Found: C, 61.39; H, 5.14; Co, 10.45; N, 5.9. UV-Vis (DMF, λmax per nm): 409, 362, 313, 266.Preparation of magnetic nanoparticles Fe3O4 (MNPs)
In order to prepare magnetite nanoparticles Fe3O4 (MNPs), a solution of FeCl2·4H2O (4 mmol, 860 mg) and FeCl3 (8 mmol, 1400 mg) in deionized water (40 mL) was stirred at 85 °C for 30 minutes under nitrogen gas. Upon addition of ammonium hydroxide (25%, 3 mL), the solution color turned from light brown to black. After stirring for 20 minutes under these conditions, the solution was cooled to room temperature followed by filtration. The magnetite precipitate was washed with H2O and sodium chloride solution (0.02 M).40Preparation of silica coated magnetite nanoparticles SCMNPs
For preparation of SCMNPs, the magnetite nanoparticles (1 g) were dispersed in deionized water in a 250 mL round-bottom flask with sonication and then an aqueous solution of TEOS (10% (v/v), 80 mL) was added, followed by glycerol (50 mL). The pH of the suspension was adjusted to 4.5 using glacial acetic acid, and the mixture was then stirred and heated at 90 °C for 2 h under a nitrogen atmosphere. After cooling to room temperature, the silica coated magnetite nanoparticles was separated from the reaction mixture using a permanent magnet and washed several times with distilled water and methanol.40Preparation of aminopropyl modified SCMNPs (Fe3O4@SiO2@APTMS)
The obtained SCMNPs (2 g) were suspended in ethanol (100 mL) and then aminopropyltrimethoxysilane (APTMS) (2 mL) was added under dry nitrogen atmosphere. The mixture was heated at reflux for 12 h and the resultant solid was magnetically separated, washed with methanol to remove the unreacted residue of silylating reagent and then dried at 80 °C.40,61Immobilization of [Co(2-hydroxy-1-naphthaldehyde)2(DMF)2] complex onto (Fe3O4@SiO2@APTMS)
To a stirring solution of complex 1 (1 mmol, 546 mg in 20 mL EtOH), Fe3O4@SiO2@APTMS (1 g) was added and the mixture heated at 90 °C for 12 h. The solid was then magnetically separated, washed with ethanol and dried at room temperature to afford Fe3O4@SiO2@APTMS@complex 1 as a brown solid and designated as catalyst 2.Catalytic oxidation, general procedure
All oxidation and epoxidation reactions were carried out in a round bottom flask equipped with a magnetic stirrer and a water-cooled condenser. Typically, catalyst (25 mg) and substrate (10 mmol) were mixed in CH3CN (5 mL). TBHP (14 mmol, 1.6 mL) was then added and the mixture was heated at reflux for several hours. After separation of the catalyst by means of an external magnet, the filtrate was subjected to GC and GC-Mass.Acknowledgements
The financial support from Alzahra University is gratefully acknowledged.References
- R. A. Sheldon and J. K. Kochi, Metal-Catalyzed Oxidations of Organic Compounds, Academic, New York, 1981 Search PubMed.
- K. A. Joergensen, Chem. Rev., 1989, 89, 431–457 CrossRef CAS.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199–218 CrossRef CAS PubMed.
- V. R. J. R. Choudhary, N. K. Chaudhari and P. Jana, Catal. Commun., 2007, 8, 1556–1560 CrossRef CAS.
- M. Beller and C. Bolm, Transition Metals for Fine Chemicals and Organic Synthesis, Wiley-VCH, Weinghem, 1998, vol. 2 Search PubMed.
- D. Chatterjee, S. Basak and J. Muzart, J. Mol. Catal. A: Chem., 2007, 271, 270–276 CrossRef CAS.
- J. Liang, Q.-H. Zhang, H.-L. Wu, G.-Y. Meng, Q.-H. Tang and Y. Wang, Catal. Commun., 2004, 5, 665–669 CrossRef CAS.
- Z. Opre, T. Mallat and A. Baiker, J. Catal., 2007, 245, 482–486 CrossRef CAS.
- T. Punniyamurthy, S. Velusamy and J. Igbal, Chem. Rev., 2005, 105, 2329–2363 CrossRef CAS PubMed.
- R. A. Budnik and J. K. Kochi, J. Org. Chem., 1976, 41, 1384–1389 CrossRef CAS.
- M. Rosaria Tine, Coord. Chem. Rev., 2012, 256, 316–327 CrossRef.
- W. Kaim and B. Schwederski, Bioinorganic Chemistry: Inorganic Elements in the Chemistry Of life, John Wiley & Sons, New York, 1993, pp. 39–51 Search PubMed.
- R. A. Budnik and J. K. Kochi, J. Org. Chem., 1976, 41, 1384–1389 CrossRef CAS.
- B. S. Garg, R. K. Sharma and E. Kundra, Transition Met. Chem., 2005, 30, 552–559 CrossRef CAS.
- L. V. Gavali and P. P. Hankare, J. Phys. Sci., 2007, 11, 147–155 Search PubMed.
- S. M. Islam, K. Ghosh, R. A. Molla, A. S. Roy, N. Salam and M. A. Iqubal, J. Organomet. Chem., 2014, 774, 61–69 CrossRef CAS.
- Y. Li, Polym. Adv. Technol., 2009, 20, 1183–1189 CrossRef.
- Y. Yoshino, Y. Hayashi and T. Iwahama, J. Org. Chem., 1997, 62, 6810–6813 CrossRef CAS.
- A. Shaabani, E. Farhangi and A. Rahmati, Appl. Catal., A, 2008, 338, 14–19 CrossRef CAS.
- Sujandi, E. A. Prasetyanto, S.-C. Han and S.-E. Park, Bull. Korean Chem. Soc., 2006, 27, 1381–1385 CrossRef CAS.
- N. C. Jana, S. Adak, P. Brandão, T. K. Mandal and A. Panja, Polyhedron, 2016, 107, 48–56 CrossRef CAS.
- T. Tezgerevska, K. G. Alley and C. Boskovic, Coord. Chem. Rev., 2014, 268, 23–40 CrossRef CAS.
- E. A. Buvaylo, A. K. Melnyk, V. V. Trachevsky, O. Y. Vassilyeva and B. W. Skelton, Polyhedron, 2016, 105, 238–245 CrossRef CAS.
- D. Brunel, N. Bellocq, P. Sutra, A. Cauvel, M. Laspéras, P. Moreau, F. D. Renzo, A. Galarneau and F. Fajula, Coord. Chem. Rev., 1998, 178–180, 1085–1108 CrossRef CAS.
- A. Choplin and F. Quignard, Coord. Chem. Rev., 1998, 178–180, 1679–1702 CrossRef CAS.
- F. Rajabi, R. Luque, J. H. Clark, B. Karimi and D. J. Macquarrie, Catal. Commun., 2011, 12, 510–513 CrossRef CAS.
- M. E. Nino, S. A. Giraldo and E. A. Paez-Mozo, J. Mol. Catal. A: Chem., 2001, 175, 139–151 CrossRef CAS.
- A. B. Sorokin, S. Mangematin and C. Pergrale, J. Mol. Catal. A: Chem., 2002, 182–183, 267–281 CrossRef CAS.
- E. Armengol, A. Corma, V. Fornes, H. Garcia and J. Primo, Appl. Catal., A, 1999, 181, 305–312 CrossRef CAS.
- Sujandi, S. C. Han, D. S. Han, M. J. Jin and S. E. Park, J. Catal., 2006, 243, 410–419 CrossRef CAS.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- L. Hamidipour, F. Farzaneh and M. Ghandi, React. Kinet., Mech. Catal., 2012, 107, 421–433 CrossRef CAS.
- C. Coperet, M. Chabanas, R. P. Saint-Arroman and J. M. Basset, Angew. Chem., Int. Ed., 2003, 42, 156–181 CrossRef CAS PubMed.
- R. N. Grass, E. K. Athanassiou and W. J. Stark, Angew. Chem., Int. Ed., 2007, 46, 4909–4912 CrossRef CAS PubMed.
- Z. Jin, Y. Luan, M. Yang, J. Tang, J. Wang, H. Gao, Y. Lub and G. Wang, RSC Adv., 2013, 1–3, 1–9 Search PubMed.
- J. Adhikary, A. Datta, S. Dasgupta, A. Chakraborty, M. Isabel Meńendez and T. Chattopadhyay, RSC Adv., 2015, 5, 92634–92647 RSC.
- G. Y. Nagesh and B. H. M. Mruthyunjayaswamy, J. Mol. Struct., 2015, 1085, 198–206 CrossRef CAS.
- S. Roy, T. N. Mandal, K. Das, R. J. Butcher, A. L. Rheingold and S. K. Kar, J. Coord. Chem., 2010, 63, 2146–2157 CrossRef CAS.
- A. B. P. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 2nd edn, 1984 Search PubMed.
- X. Liu, Z. Ma, J. Xing and H. Liu, J. Magn. Magn. Mater., 2004, 270, 1–6 CrossRef CAS.
- J. Wang, S. Zheng, Y. Shao, J. Liu, Z. Xu and D. Zhu, J. Colloid Interface Sci., 2010, 349, 293–299 CrossRef CAS PubMed.
- L. Hamidipour, F. Farzaneh and M. Ghandi, React. Kinet., Mech. Catal., 2012, 107, 421–433 CrossRef CAS.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS PubMed.
- M. Masteri-Farahani and Z. Kashef, J. Magn. Magn. Mater., 2012, 324, 1431–1434 CrossRef CAS.
- Y. Guo, Z. Liu, G. Wang, Y. Huang and F. Kang, Appl. Surf. Sci., 2011, 258, 1082–1090 CrossRef CAS.
- Q. H. Tang, Q. H. Zhang, H. L. Wu and Y. Wang, J. Catal., 2005, 230, 384–397 CrossRef CAS.
- J. Liu, F. Wang, S. Qi, Z. Gu and G. Wu, New J. Chem., 2013, 37, 769–774 RSC.
- C. Saux and L. B. Pierella, Appl. Catal., A, 2011, 400, 117–121 CrossRef CAS.
- S. M. Islam, A. S. Roy, P. Mondal, S. Mondal, M. Mubarak, D. Hossain and S. Sarkar, J. Appl. Polym. Sci., 2011, 120, 2743–2753 CrossRef CAS.
- R. Rinaldi, F. Y. Fujiwara, W. Hölderich and U. Schuchardt, J. Catal., 2006, 244, 92–101 CrossRef CAS.
- M. R. dos Santos, J. R. Diniz, A. M. Arouca, A. F. Gomes, K. A. Srinivas, A. Kumar and S. M. S. Chauhan, Chem. Commun., 2002, 2456–2457 Search PubMed.
- F. C. Gozzo, S. M. Tamborim, A. L. Parize, P. A. Z. Suarez and B. A. D. Neto, ChemSusChem, 2012, 5, 716–726 CrossRef PubMed.
- J. Zhang, A. V. Biradar, S. Pramanik, T. J. Emge, T. Asefa and J. Li, Chem. Commun., 2012, 48, 6541–6543 RSC.
- L. M. Slaughter, J. P. Collman, T. A. Eberspacher and J. I. Brauman, Inorg. Chem., 2004, 43, 5198–5204 CrossRef CAS PubMed.
- F. Farzaneh, J. Taghavi, R. Malakooti and M. Ghandi, J. Mol. Catal. A: Chem., 2006, 244, 252–257 CrossRef CAS.
- J. Zhang, A. V. Biradar, S. Pramanik, T. J. Emge, T. Asefa and J. Li, Chem. Commun., 2012, 48, 6541–6543 RSC.
- Z. Guo, C. Zhou, S. Hu, Y. Chen, X. Jia, R. Lau and Y. Yang, Appl. Catal., A, 2012, 419–420, 194–202 CrossRef CAS.
- M. Reichinger, W. Schmidt, M. W. E. v. d. Berg, A. Aerts, J. A. Martens, C. E. A. Kirschhock, H. Gies and W. Grünert, J. Catal., 2010, 269, 367–375 CrossRef CAS.
- M. Lenze and E. B. Bauer, J. Mol. Catal. A: Chem., 2009, 309, 117–123 CrossRef CAS.
- U. Neuenschwander and I. Hermans, J. Org. Chem., 2011, 76, 10236–10240 CrossRef CAS PubMed.
- E. L. Eliel and S. H. Wilen, Stereochemistry of Organic Compounds, John Wiley & Sons, Inc., 1994, New York, p. 726 Search PubMed.
- J. March, Advanced Organic Chemistry, John Wiley & Sons, Inc, New York, 3rd edn, 1985, p. 616 Search PubMed.
- M. Mohammadikish, M. Masteri-Farahani and S. Mahdavi, J. Magn. Magn. Mater., 2014, 354, 317–323 CrossRef CAS.
- G. Sheldrick, SHELX-97, Program for the solution and refinement of crystal structures, University of Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1403895. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra18154f |
| This journal is © The Royal Society of Chemistry 2016 |