Harvesting red fluorescence through design specific tuning of ICT and ESIPT: an efficient optical detection of cysteine and live cell imaging†
Abstract
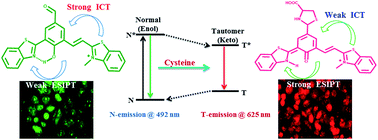
In this report, we constructed 2-(2-hydroxyphenyl)benzothiazole (HBT) based ratiometric fluorescent probe R1, highly selective for cysteine (Cys) in the red channel with a nanomolar detection limit. The rational combination of benzothiazole and benzothiazolium unit within the same molecular frame work act as “two-heads” that allowed the simultaneous harvesting of intramolecular charge transfer (ICT) as well as excited state intramolecular proton transfer (ESIPT). This coupling of dual mechanism in R1 provides selective colorimetric and ratiometric fluorescence response towards Cys from the mixture of various anions and amino acids including foremost interfering thiols viz. HS−, Hcy and GSH. R1 shows a weak ESIPT at 497 nm (green fluorescence, N*) which undergoes shifting to 625 nm (red channel K*) upon addition of Cys selectively. Henceforth, R1 was also utilized for marking Cys in live cell imaging in HeLa cells. The sensing phenomenon of R1 for Cys was studied through a number of spectroscopic techniques viz., NMR, IR along with HRMS studies. The density functional theory calculations further strengthened the experimental findings.

 Please wait while we load your content...
Please wait while we load your content...