Structural evolution of palladium nanoparticles and their electrocatalytic activity toward ethanol oxidation in alkaline solution†
Abstract
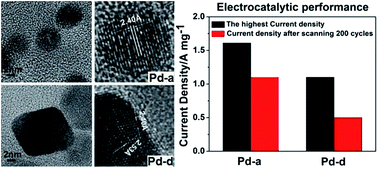
A series of palladium nanoparticles with sizes ranging from 3 to 10 nm were synthesized with ethylene glycol as the solvent and reducing agent. Based on X-ray diffraction, transmission electron microscopy and high-resolution transmission electron microscopy characterization, the as-prepared palladium nanoparticles were well-crystalline and uniformly dispersed. Electrochemical measurements showed that the smallest (∼3 nm) palladium nanoparticles exhibited the highest current density and the best cycling stability and durability among the four kinds of palladium nanoparticles for the electrocatalytic oxidation of ethanol. In addition, small palladium nanoparticles were spherical while large nanoparticles with a certain decline in electrocatalytic activity showed distinct shape. The formation and shape evolution of the palladium nanoparticles as well as their structure–property relationship have been studied and analyzed based on the experimental data.

 Please wait while we load your content...
Please wait while we load your content...