129Xe NMR studies of morphology and accessibility in porous biochar from almond shells†
M. Farinaa,
M. Mauri *ab,
G. Patriarcaa,
R. Simonuttiab,
K. T. Klassonc and
H. N. Cheng*c
*ab,
G. Patriarcaa,
R. Simonuttiab,
K. T. Klassonc and
H. N. Cheng*c
aDepartment of Materials Science, University of Milan-Bicocca, via R. Cozzi 55, 20125 Milan, Italy. E-mail: michele.mauri@mater.unimib.it
bINSTM, via Giuseppe Giusti 9, 50121, Firenze, Italy
cUSDA Agricultural Research Service, Southern Regional Research Center, 1100 Robert E. Lee Blvd., New Orleans, LA 70124, USA. E-mail: HN.Cheng@ARS.USDA.GOV
First published on 25th October 2016
Abstract
Micro and mesoporous materials are often used in catalysis, purification, composite filler, and other applications. Almond shell is an important agricultural byproduct that can be transformed to microporous and mesoporous carbon. In this work, we produced biochar from almond shell using a thermal treatment procedure in an inert atmosphere and characterized the pores with nitrogen adsorption, environmental SEM, and 129Xe NMR. The latter technique differentiates adsorbed and nonadsorbed xenon and permits the correlation of different processing conditions with xenon adsorption and diffusivity. The relevance of removing the ash produced during the charring process has been included in the study. Moreover, the xenon exchange between meso- and micro-pores has been directly observed by 129Xe NMR, demonstrating that after ash removal by water the materials have high accessibility of the pores by external fluids, thus increasing the usefulness as filtration or adsorption material.
Introduction
Significant amounts of shells are inevitably associated with the production of almond nuts for human consumption. Shells are made up of biopolymers such as cellulose, hemicellulose, pectin, and lignin1 and use of this industrial byproduct constitutes an opportunity for sustainable development. Currently almond shells are often utilized as animal feed, as landscaping material, or incinerated for energy production. However, the chemical and physical characteristics of the shell structure provide opportunities for alternative uses. Some additional applications of almond shells include the production of furfural,2 xylan antioxidants1 and substrates in sweet pepper production.3 Yet another significant application is the production of biochar, a pyrolyzed carbonaceous material that partly conserves the complex structure due to the phytotomy of the shells.4–8Biochars belong to the class of micro- and meso-porous materials that have found utility in catalysis, purification, composite fillers, water treatment,9 soil conditioning,10 and even reversal of climate change11 or nanodevices.12 Almond shell is a material with abundant supply and low value, while porous materials are in high demand due to a variety of applications. Biochar can be made by activating almond shells with a number of processes, such as high temperature without oxygen,4,5 low temperature in the presence of ammonium chloride,13 and a two-step process first involving heating in nitrogen at one temperature and then heating in CO2 at a different temperature.6,7 The biochar from almond shells is being considered for use in removal of impurities and toxic organic compounds from water and industrial streams6,14 and mercury removal in flue gas.5 On a first approximation, the end-use properties are affected by the porosity of biochar, but the details of the carbonization process are complex, especially considering that the pores formed can be blocked by concurrently generated ash or tar. Certainly a better understanding of void structure and formation is useful.15
Environmental scanning electron microscopy (ESEM) and 129Xe nuclear magnetic resonance (NMR) are good methods for a morphological characterization of biochar samples that provide information simultaneously on pore size and accessibility of the porous systems at different size-scales. Particularly, ESEM can provide images down to the hundreds of nanometers without requiring the high vacuum conditions that may alter samples in conventional SEM microscopy. 129Xe NMR allows investigating smaller pores down to a single nm. More precisely, xenon chemical shift is related to free mean path of xenon atoms in a given environment, and ultimately to pore size.16 Thus, as recently reviewed,17 the latter technique has proven itself as an effective tool to characterize several systems, such as well-ordered zeolites18 but also minerals, clays and soils.19–25 The use of xenon, which is mostly lipophilic, is a good model for the adsorption of organic contaminants, such as the diffusion of organic monomers in olefins or methane in porous systems.26 129Xe NMR has been used to study carbonaceous materials27 including recent studies on biochar from pecan shells.28 In order to extend the latter work, we have taken a closer look at almond shell biochar with ESEM and 129Xe NMR and followed an integrated approach where chemical shift measurement is complemented by advanced 2D NMR techniques that provide the direct detection of xenon exchange within the biochar.
Experimental section
Almond shell preparation
Almond shell biochar was prepared by treating almond shell in an inert atmosphere at 800 °C. A portion of each sample was also set aside and washed with synthetic rain water at room temperature for 20 hours in a tumbler. The synthetic rainwater was made by adding a mixture of sulfuric and nitric acids (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 wt%) to distilled water until the pH was 4.2 (±0.05).29 After the rainwater wash, each sample was then sieved to remove the water through a 400-mesh (37 μm) screen. It was possible that some fine soot might have been lost during sifting. Washed samples were then dried overnight in an oven at 100 °C. It was a very mild process in comparison with the charring process and with the preparation of Xe tubes described below, and we assume it did not affect sample morphology. After treatment, all samples appear as black powder, made of particles less than 2 mm across.
40 wt%) to distilled water until the pH was 4.2 (±0.05).29 After the rainwater wash, each sample was then sieved to remove the water through a 400-mesh (37 μm) screen. It was possible that some fine soot might have been lost during sifting. Washed samples were then dried overnight in an oven at 100 °C. It was a very mild process in comparison with the charring process and with the preparation of Xe tubes described below, and we assume it did not affect sample morphology. After treatment, all samples appear as black powder, made of particles less than 2 mm across.
N2 BET porosimetry
The Brunauer–Emmet–Teller (BET) specific surface area was measured using a NOVA 2200e nitrogen adsorption system (Quantachrome Instruments, Boynton Beach, FL, USA). These values were used to calculate the average cylindrical pore diameter, assuming that the total pore volume was occupied by liquid nitrogen at atmospheric pressure and −196 °C.Environmental scanning electron microscopy
Environmental scanning electron microscopy (ESEM) was used to visualize the morphology of the biochar samples. The instrument was a XL-30 environmental scanning electron microscope (FEI, Hillsboro, OR, USA). The biochars were mounted on aluminum specimen stubs with two-sided adhesive tapes (Electron Microscopy Sciences, Hatfield, PA, USA) and sputter coated with 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 gold
40 gold![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) palladium to a thickness of 10–15 nm. Standard operating procedures were followed in obtaining the electron photomicrographs (10.0 kV voltage).
palladium to a thickness of 10–15 nm. Standard operating procedures were followed in obtaining the electron photomicrographs (10.0 kV voltage).
129Xe NMR spectroscopy
129Xe NMR spectroscopy was performed at 25 °C, using a Bruker Avance 500 spectrometer equipped with a 10 mm probe with 129Xe resonance at 138.45 MHz. The gas employed (Xenon 5.0, Sapio S.r.l., Italy) had a nominal content of 26.44% (natural abundance) of the 129Xe isotope.Around 250 mg of almond-shell-based biochar particles were loosely packed in a heavy-walled 10 mm NMR tube (with 7 mm i.d.) in the field-sensitive part of the NMR coil. Atmospheric gases were removed by applying a dynamic vacuum (<10−1 mbar) on the tube overnight at room temperature or at 180 °C, using a rotary mechanical pump with nitrogen trap. The xenon gas was transferred quantitatively from a reservoir of known volume (28.3 mL) into the sample tube via a Schlenk manifold by freezing the bottom of the tube with liquid nitrogen. The tube with xenon and the sample was hermetically sealed with a flame. One-dimensional (1D) spectra with 5 bar xenon loading were acquired with single pulse excitations using 32 μs π/2 pulse length, 200 scans and recycle delays of 4 s. Low pressure studies required a correspondingly increased number of scans in order to maintain acceptable S/N ratios: 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 scans were needed to collect the NMR spectra at 0.5 bar. For exchange studies, a pressure of 5 bars was used, ensuring sufficient signal-to-noise (S/N) ratio for performing the long two-dimensional (2D) exchange (EXSY) experiments in a reasonable amount of time. The EXSY experiments were run with a recycle delay of several seconds and a spectral width of 300 ppm in both t1 and t2 dimensions. There were 128 t1 increments. 2D data were collected in TPPI mode. Mixing times were varied from 20 to 1000 ms. Longitudinal relaxation time T1 was measured on the same 5 bar samples using standard saturation recovery sequence and 12 data points.
000 scans were needed to collect the NMR spectra at 0.5 bar. For exchange studies, a pressure of 5 bars was used, ensuring sufficient signal-to-noise (S/N) ratio for performing the long two-dimensional (2D) exchange (EXSY) experiments in a reasonable amount of time. The EXSY experiments were run with a recycle delay of several seconds and a spectral width of 300 ppm in both t1 and t2 dimensions. There were 128 t1 increments. 2D data were collected in TPPI mode. Mixing times were varied from 20 to 1000 ms. Longitudinal relaxation time T1 was measured on the same 5 bar samples using standard saturation recovery sequence and 12 data points.
Results and discussion
Sample description, BET and N2 data
Table 1 presents the samples used in this work: they were labelled according to the duration of the thermal treatment and whether they were also washed with water. BET specific surface areas and calculated N2 average pore diameters (cylindrical model) are reported as well. Note that the 800 °C thermal treatment increases the porosity by two orders of magnitude, and the water wash increases porosity even further.| Sample label | 800 °C thermal treatment duration (min) | Additional treatment | BET N2 specific surface area (m2 g−1) | N2 pore diameter (Å) |
|---|---|---|---|---|
| 60 | 60 | — | 2 ± 2 | — |
| 120 | 120 | — | 81 ± 5 | 10.1 ± 0.4 |
| 240 | 240 | — | 201 ± 9 | 9.3 ± 0.2 |
| 60 W | 60 | Washed with water | 2 ± 2 | — |
| 120 W | 120 | Washed with water | 311 ± 30 | 9.5 ± 0.5 |
| 240 W | 240 | Washed with water | 493 ± 40 | 9.1 ± 0.2 |
Morphological investigation by environmental scanning electron microscopy
Fig. 1 shows representative ESEM micrographs of samples 240 and 240 W at two different magnifications (500× and 5000×). At the lower magnification, samples show a structure with pores in the micrometre range. Increased magnification shows that the walls are patterned with a second level of structure: large mesopores in the submicrometric range. In the unwashed sample (top pictures) part of the macropores are obstructed by dust, ash, or inorganic salts, and even in the regions where their walls can be observed directly the image appears opaque, as if overlaid by a thin layer. Washing with water removes the dust and the salt, fully revealing the pores of the beehive-like structure (bottom pictures) with a wide range of sizes. At the higher magnification, the mesopores in the washed sample also appear deeper and better defined. | ||
| Fig. 1 ESEM micrographs of almond shell biochar prepared with 240 min thermal treatment at two different magnifications (500× and 5000×). Top: sample 240. Bottom: sample 240 W. | ||
129Xe correlation of data with process parameters and N2 BET data
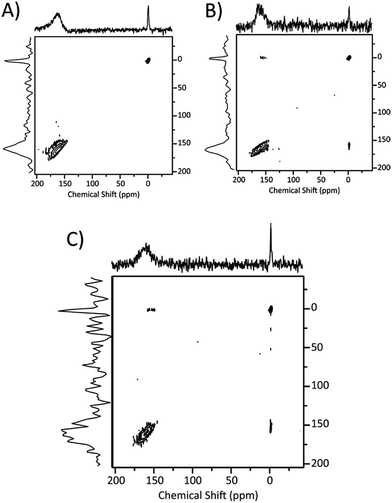
129Xe NMR data for the six samples of almond shell biochar are presented in Fig. 2 and Table 2. Spectra of unwashed samples are shown on the left in Fig. 2. At the bottom left, the spectrum of sample 60 displays a single peak, located near 0 ppm and with a linewidth (LW) of 278 Hz (1.87 ppm). An error of ±0.1 ppm is estimated for this peak. The small chemical shift and linewidth are typically associated with gaseous or nonadsorbed xenon. Furthermore, the measured longitudinal relaxation time T1 is close to 10 seconds, a typical value for free xenon gas confined in a NMR tube. Thus, the spectrum is clearly associated with a system where porosity is negligible or inaccessible. Since the chemical shift of xenon is known to have a pressure dependence that is linear up to 100 amagats30 and peaks with similar shift and LW appear in all the 129Xe NMR spectra in biochar, the nonadsorbed xenon gas provides an internal standard for determining the chemical shifts in biochars, as demonstrated in our previous work in pecan shell char.28| Sample | Sample pressure (bar) | Adsorbed xenon chemical shift (ppm) | NMR LW (ppm) | NMR area (norm. integral) | T1 gas (s) | T1 pores (s) |
|---|---|---|---|---|---|---|
| 60 | 5.02 | — | — | — | 9.2 | — |
| 120 | 5.02 | 166 | 20.5 | 1.87 | — | — |
| 240 | 5.07 | 154 | 26.1 | 2.44 | — | — |
| 60 W | 5.04 | 183 | 18.0 | 1.15 | 5.1 | 1.9 |
| 120 W | 4.90 | 163 | 18.0 | 5.11 | 4.0 | 2.3 |
| 240 W | 5.01 | 158 | 22.9 | 6.08 | 2.8 | 2.6 |
With a longer thermal treatment time (120 min), a second signal appears downfield at around 166 ppm. This signal has a much greater linewidth (20.5 ppm), and the error on its chemical shift is estimated as ±1 ppm. Since chemical shift is related to pore size,17 this spectrum is evidence of porosity with a relatively wide pore size distribution. With a longer treatment time (240 min), this broad signal shifts slightly upfield to 157 ppm and becomes somewhat broader in linewidth (26 ppm). While the ratio between the integrated areas of the two peaks in sample 120 and 240 is not completely quantitative due to incomplete relaxation and the difficulty in defining actual free volume for the coarse powder, the accessible volume appears to be similar and quite stable.
The impact of washing with water is shown by comparison with the three spectra on the right in Fig. 2. The water wash has a small effect on the chemical shift, but a much greater effect on signal intensity. Samples 120 W and 240 W present similar chemical shift (2–3 ppm difference, which is reasonable considering the line width) relative to their unwashed counterparts. The peak of sample 240 W also changes in shape: it is broader and asymmetric, with a tail extending to smaller chemical shifts. Sample 120 W shows the same behaviour in a less evident way. A satisfactory line fitting of both the 120 W and 240 W spectra could be performed with two separate and broad peaks (see ESI†), indicating the presence of pores with different sizes, and a complex pattern of gas exchange. Lastly, in both samples the intensities of the adsorbed xenon signals increase notably when compared to the spectra of unwashed samples: since the peak intensity and integrated area can be correlated to the amount of porosity, the water wash clears up more pores which thus become 129Xe accessible. Note that the linewidth also increases with longer heating, suggesting the occurrence of a broader distribution of pore sizes.
Interestingly, the spectrum of sample 60 W also displays a signal associated to the pores that was completely absent in the unwashed counterpart. The chemical shift (183 ppm) suggests that the newly formed pores are relatively smaller in size than those in the other samples. Pore accessibility is also suggested by the T1 data reported in the last column of Table 2: in sample 60 W, the two peaks display starkly different relaxation times: around 5 seconds for the peak associated with nonadsorbed xenon and 2 seconds for the adsorbed gas. Higher values are typical of nonadsorbed gas, and it is interesting to note that by increasing thermal treatment time as in sample 240 W the two values tend to converge, indicating an exchange between the two environments.
The 129Xe NMR data are generally consistent with N2 BET surface area data, reported in Table 1. Indeed, N2 BET results also indicate that the biochar has negligible porosity up to 60 min thermal treatment, but as a result of further heat treatment, surface area increases significantly from 2 to 201 m2 g−1. Also, the water wash makes accessible some previously covered porosity in the biochar, with an increase of a factor 2 or more in the quantified specific surface areas of samples with the same duration of thermal activation. Whereas N2 BET indicates an almost insignificant amount of porosity for both samples 60 and 60 W, 129Xe NMR can detect porosity in the washed sample 60 W. In other words, 129Xe NMR is qualitatively more sensitive to porosity below the minimum sensitivity of the BET technique for sample 60 W.
Dependence of 129Xe chemical shifts with pressure
As in the previous work,28 we have studied the 129Xe chemical shifts versus xenon gas pressure, with the aim of extrapolating the chemical shift at 0 bar pressure, a value that can be connected to the mean free path Γ of confined xenon through Fraissard and Demarquay's equation,where δXe is the extrapolated zero-pressure chemical shift.14,15 The average effective pore size can be calculated by choosing an appropriate geometrical model for the pores. Throughout the paper, we used a cylindrical model.
The results for samples 240 and 240 W are plotted in Fig. 3. Contrary to what was observed for pecan shell-based biochars,21 the shift versus pressure plot is not linear, but slightly curved suggesting a sublinear behaviour, especially at higher pressures. Previously Oschatz et al.31 studied several micro- and mesoporous carbon materials at high pressure and also observed such non-linear behavior for some of the materials: after an initial increase, 129Xe chemical shift levelled off and reached a plateau. In accordance with Oschatz, the curve can be interpreted as due to xenon being in a dynamic isothermal adsorption/desorption state. Intuitively, linearity is due to Xe–Xe interaction and is thus typical of systems where a pressure increase corresponds to a linear increase of xenon density within the pores, as in zeolites. Instead, when there is a dynamic equilibrium with a highly accessible system, increased gas density can be accommodated only partially on the limited pore surface, where it behaves in increasingly higher proportion as nonadsorbed gas, providing a sublinear increase of chemical shift as seen in Fig. 3.
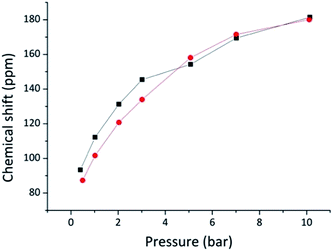 | ||
| Fig. 3 Plot of the dependence of 129Xe chemical shift on pressure. Squares represent sample 240 and circles represent sample 240 W. | ||
Nevertheless, in the low pressure limit (≤2 bar), xenon is far from being saturated but mostly adsorbed on the surface: the chemical shift pressure dependence is almost linear and thus Fraissard and Demarquay's equation16 can be used to provide a 0 bar chemical shift extrapolation for average pore size calculation through a cylindrical model (Table 3). The values obtained directly from the ca. 0.5 bar sample chemical shifts were also used to provide an alternative estimate with slightly smaller average pore sizes and should be considered lower bounds. The results of both methods are presented in Table 3, and together they provide an estimate of the uncertainty in pore size measured by 129Xe NMR. The sample washed with water presents slightly larger pores than the unwashed sample, but the difference is small and approaches the limit of experimental error.
| Sample | Pressure (bar) | Chemical shift (ppm) | Shift at zero pressure (ppm) | Mean free path (Å) | Calculated average pore size-cylindrical model (Å) |
|---|---|---|---|---|---|
| 240 | 0.40 | 93 | 84 | 3.3–3.9 | 7.7–8.3 |
| 1.01 | 112 | ||||
| 2.03 | 131 | ||||
| 3.02 | 145 | ||||
| 5.07 | 154 | ||||
| 7.02 | 169 | ||||
| 10.14 | 181.5 | ||||
| 240 W | 0.49 | 87 | 80 | 3.7–4.2 | 8.1–8.6 |
| 1.02 | 102 | ||||
| 2.03 | 121 | ||||
| 3.02 | 134 | ||||
| 5.07 | 158 | ||||
| 7.01 | 171.5 | ||||
| 10.11 | 180 |
Exchange between nonadsorbed and adsorbed xenon
Since the variable pressure chemical shift measurements and the chemical shift lineshapes both indicated the presence of exchange phenomena within the pores and between the pores and the external environment, we investigated them directly using the EXSY sequence32 with variable exchange time. 2D spectra acquired with this technique exhibit off-diagonal peaks with intensities depending on the actual exchange taking place during the set time. Of course, sample 60 has no signal except for the region around 0 ppm, associated with nonadsorbed gas; thus no exchange spectroscopy could be performed. The corresponding water-washed sample (60 W) instead presents two separate signals, so we tested it with several different exchange times (20 ms, 400 ms, 1000 ms): all those exchange times show analogous 2D spectra with no detectable peaks outside the diagonal. Longer times could not be efficiently probed, due to the intrinsic limitation of T1 relaxation that reduces S/N ratio: as indicated in Table 2, T1 relaxation time for xenon associated with the pore systems is around 2 s. Still, the exchange times allowed demonstrate that there is no significant xenon exchange between adsorbed and nonadsorbed peaks in this sample up to the half-second range. As we look at samples with progressively longer thermal treatment times, sample 120 also provides a spectrum without cross-peaks, indicating low xenon accessibility as in the samples prepared with 60 minutes thermal treatment. Instead, sample 120 W is the first washed sample which shows xenon exchange, as depicted in Fig. 4. All visible peaks at 20 ms exchange time are found on the diagonal. At 400 and 1000 ms exchange times, non-diagonal peaks appear, indicating exchange between the adsorbed and the nonadsorbed peaks (Fig. 4). Additionally, the diagonal peaks at 150 ppm are relatively broad (130–175 ppm), suggesting some internal xenon exchange between different pore systems in the material. Only the xenon at lower chemical shifts (i.e., larger pores) exchange with the nonadsorbed gas: thus, larger pores are more easily connected with the external environment.Since 400 ms exchange time appears to be a good compromise between high exchange rate and sufficient S/N ratio considering the longitudinal relaxation, further experiments at 400 ms exchange time were carried out on samples 120, 240 and their water-washed counterparts. A comparison of the EXSY plots is shown in Fig. 5. For both samples 120 and 240, only diagonal peaks are found. However, with samples 120 W and 240 W, some non-diagonal elements are clearly evident indicating xenon exchange between the adsorbed and nonadsorbed xenon. Sample 240 W displays the greater exchange, as indicated by the high relative intensity of the off-diagonal peaks. Note that the diagonal peaks are large and show up more like a cluster, suggesting that some internal xenon exchange among pores is taking place as well. Thus, the water wash treatment has a significant role in increasing the accessible pores volumes and allowing the xenon to exchange.
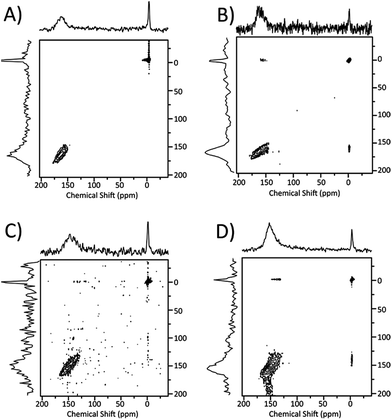 | ||
| Fig. 5 2D EXSY spectra for sample 120 (A), 120 W (B), 240 (C), 240 W (D) (5 bar) at 400 ms exchange time. | ||
Pore structure and accessibility
Upon combining 129Xe NMR and N2 BET data, a picture emerges of the effects of thermal treatment and water wash on almond shell biochar at the submicrometer scale. After heating at 800 °C for 60 minutes (sample 60), degradation occurs in the almond shell but pores are either not well-formed or filled with soot and salt, and thus not detected by 129Xe NMR. With water wash (sample 60 W), the soot and salts removal opens up pores with relatively small sizes, as indicated by the NMR data. After thermal treatment for 120 minutes (sample 120), either more or slightly wider pores are formed according to 129Xe NMR and N2 BET data. With water wash (sample 120 W), total pore volume increases while the average pore size stays the same; thus, water wash opens up pores similar to the ones already accessible. Increased thermal treatment from 120 to 240 minutes produces a sample (sample 240) with the adsorbed peak at a slightly smaller chemical shift but an increased peak area and broader linewidth; thus, the pore volume increases, but new small pores are formed and existing pores get larger, probably due to the breakdown or thinning of the interpore walls. With water wash (sample 240 W), the pore volume increases further but the average pore size stays the same. If one compares the data for samples 120 and 240, the average pore size for samples 240 is slightly larger according to NMR chemical shifts but slightly smaller according to nitrogen pore diameter data. If we compare samples 240 and 240 W, the average pore dimensions as calculated from N2 BET and 129Xe NMR through Fraissard and Demarquay's equation also show different relative trends. However, these differences are very small, some of which approach the limits of experimental error. These differences may perhaps also reflect the different sensitivities of 129Xe NMR and N2 BET to the broad pore size distributions and 129Xe adsorption/desorption behaviour in almond shell biochars. The broad lines are associated to pore size distribution rather than to fast T2 relaxation since the latter is usually associated to the presence of significant amounts of paramagnetic particles such as residual catalyst in carbon nanotubes33 or iron oxide in building stone materials.34 Previous investigations on the composition of biochar from nut shells do not indicate the presence of such defects.On the basis of the results reported here and of an earlier model,35 an idealized representation of the pore structure of biochar particles can be envisaged (Fig. 6). Locally, they are composed by graphitic layers interconnected in disordered fashion and forming roughly cylindrical channels of about 8 Å (twice as large as the xenon atom, 4.4 Å). Microporous aggregates of graphitic layers are then arranged in mesopores, where xenon can exchange further with the macropores or the interparticle space.
Conclusions
In this work, we investigated the morphology of biochar samples prepared by activating almond shell in inert atmosphere at 800 °C. Macropores in the tens of micron range with walls decorated by a second level of submicrometric mesopores are formed by the heating treatment, as evidenced by ESEM micrographs. A third level of even smaller micropores was studied by N2 BET and 129Xe NMR. For most conditions, including some where porosity was not detected by N2 BET, we found two 129Xe NMR signals, one associated with micropores with average diameter of around 8 Å, and one associated with the non-adsorbed gas residing in all other meso and macropores where xenon is not strongly affected by confinement. Moreover, the signal associated with the micropores is in internal and external exchange as demonstrated by sublinearity of 129Xe shift as a function of pressure. In the (unwashed) thermally treated samples, there is no exchange visible by EXSY between the two peaks. Exchange instead appears upon washing samples with artificial rainwater. To understand this complex picture, we hypothesise two different populations of NMR-detectable xenon atoms. The first consists of gas exchanging very fast in the NMR timescale concentrated in the immediate vicinity of the surface, possibly within the mesopores seen on the walls of the biochar material by microscopy. This population is in exchange with nonadsorbed gas, either outside the granules or within the macropores. The nonadsorbed gas is independent of the gas in the mesopores even in the tenths of second timescale in the unwashed samples. Water treatment clears the pores up from soot and salts, opening up more confined environment (micropores) where the second population of xenon is found. The xenon exchange between the first and the second population has larger mean free paths and basically indicates accessibility. It is potentially the most important for actual applications, since it describes the possibility for an external fluid to permeate throughout the material. In an application like filtration, adsorption, or organic contaminant removal, the substrate molecule may adsorb on the surface of biochar particles or diffuse into the pores. Exchange among the substrate molecules in different environments may occur, similar to what xenon does. 129Xe NMR therefore may provide useful insight on biochar–substrate interactions.Acknowledgements
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer. M. F. thanks CORIMAV for financial support through PCAM European Doctoral Program.References
- A. Ebringerová, Z. Hromádková, Z. Košt'álová and V. Sasinková, BioResources, 2008, 3, 60 Search PubMed.
- A. Demirbasa, Energy Sources, Part A, 2006, 28, 157 CrossRef.
- M. Valverde, R. Madrid, A. L. García, F. M. del Amor and L. Rincón, Spanish J. Agr. Res., 2013, 11, 164 CrossRef.
- K. T. Klasson, M. Uchimiya and I. M. Lima, Ind. Crops Prod., 2015, 67, 33 CrossRef CAS.
- K. T. Klasson, M. Uchimiya and I. M. Lima, Chemosphere, 2014, 11, 129 CrossRef PubMed.
- A. Omri, M. Benzina and N. Ammar, J. Ind. Eng. Chem., 2013, 19, 2092 CrossRef CAS.
- J. M. V. Nabais, C. E. C. Laginhas, P. J. M. Carrott and M. M. L. R. Carrott, Fuel Process. Technol., 2011, 92, 234 CrossRef CAS.
- R. Bansode, J. N. Losso, W. E. Marshall, R. M. Rao and R. J. Portier, Bioresour. Technol., 2003, 90, 175 CrossRef CAS PubMed.
- S. C. Peterson, M. A. Jackson and M. Appell, ACS Symp. Ser., 2013, 1143, 193 CrossRef CAS.
- S. P. Sohi, E. Krull, E. Lopez-Capel and R. Bol, Adv. Agron., 2010, 105, 47 CAS.
- J. Lehmann, Front. Ecol. Environ., 2007, 5, 381 CrossRef.
- E. Redondo, J. Carretero-González, E. Goikolea, J. Ségalini and R. Mysyk, Electrochim. Acta, 2015, 160, 178 CrossRef CAS.
- S. Balci, T. Dogu and H. Yucel, J. Chem. Technol. Biotechnol., 1994, 60, 419 CrossRef CAS.
- K. T. Klasson, C. A. Ledbetter, M. Uchimiya and I. M. Lima, Environ. Chem. Lett., 2013, 11, 271 CrossRef CAS.
- S. Joseph, C. Peacocke, J. Lehmann and P. Munroe, Biochar for Environmental Management Science and Technology, ed. J. Lehmann and S. Joseph, Earthscan, Abington, Oxon, UK, 2009, pp. 107–126 Search PubMed.
- J. Demarquay and J. Fraissard, Chem. Phys. Lett., 1987, 136, 314 CrossRef CAS.
- E. Weiland, M.-A. Springuel-Huet, A. Nossov and A. Gédéon, Microporous Mesoporous Mater., 2016, 225, 41–65 CrossRef CAS.
- T. Ito and J. Fraissard, J. Chem. Phys., 1982, 76, 5225 CrossRef CAS.
- D. Raftery, Annu. Rep. NMR Spectrosc., 2006, 57, 205 CrossRef CAS.
- C. Dybowski, N. Bansal and T. M. Duncan, Annu. Rev. Phys. Chem., 1991, 42, 433 CrossRef CAS.
- P. Sozzani, S. Bracco, A. Comotti, M. Mauri, R. Simonutti and P. Valsesia, Chem. Commun., 2006, 18, 1921 RSC.
- S. Bracco, P. Valsesia, L. Ferretti, P. Sozzani, M. Mauri and A. Comotti, Microporous Mesoporous Mater., 2008, 107, 102 CrossRef CAS.
- R. W. Mair, G. P. Wong, D. Hoffmann, M. D. Hürlimann, S. Patz, L. M. Schwartz and R. L. Walsworth, Phys. Rev. Lett., 1999, 83, 3324 CrossRef CAS PubMed.
- Y. Peng, V. Krungleviciute, I. Eryazici, J. T. Hupp, O. K. Farha and T. Yildirim, J. Am. Chem. Soc., 2013, 135, 11887 CrossRef CAS PubMed.
- J. A. Mason, M. Veenstra and J. R. Long, Chem. Sci., 2014, 5, 32 RSC; S. Jost, S. Fritzsche, R. Haberlandt and J. Karger, J. Phys. Chem. B, 1998, 102, 6375 CrossRef CAS.
- A. Comotti, S. Bracco, P. Valsesia, L. Ferretti and P. Sozzani, J. Am. Chem. Soc., 2007, 129, 8566 CrossRef CAS PubMed.
- X. Zhu, I. L. Moudrakovski and J. A. Ripmeester, Energy Fuels, 1997, 11, 245 CrossRef CAS; D. J. Suh, T. J. Park, S. K. Ihm and R. Ryoo, J. Phys. Chem., 1991, 95, 3767 CrossRef.
- M. Mauri, M. Farina, G. Patriarca, R. Simonutti, K. T. Klasson and H. N. Cheng, Int. J. Polym. Anal. Charact., 2015, 20, 119 CrossRef CAS.
- Anon, Method 1312 Synthetic precipitation leaching procedure. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, U.S. Environmental Protection Agency, Cincinnati, OH, USA, 1994 Search PubMed.
- A. K. Jameson, J. Chem. Phys., 1970, 53, 2310 CrossRef CAS.
- M. Oschatz, H. C. Hoffmann, J. Pallmann, J. Schaber, L. Borchardt, W. Nickel, I. Senkovska, S. Rico-Franceés, J. Silvestre-Albero, S. Kaskelband and E. Brunner, Chem. Mater., 2014, 26, 3280 CrossRef CAS.
- J. Jeener, B. H. Meier, P. Bachmann and R. R. Ernst, J. Chem. Phys., 1979, 71, 4546 CrossRef CAS .
- C. F. M. Clewett and T. Pietraβ, J. Phys. Chem. B, 2005, 109, 17907–17912 CrossRef CAS PubMed.
- M. Mauri and R. Simonutti, Materials, 2012, 5, 1722–1739 CrossRef CAS.
- F. Rodriguez-Reeinoso, Carbon, 1998, 36, 159 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18104j |
| This journal is © The Royal Society of Chemistry 2016 |