Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, structure and photochromic properties of hybrid molecules based on fullerene C60 and spiropyrans†
A. R.
Tuktarov
*a,
A. A.
Khuzin
a,
A. R.
Tulyabaev
 a,
O. V.
Venidictova
b,
T. M.
Valova
b,
V. A.
Barachevsky
b,
L. M.
Khalilov
a and
U. M.
Dzhemilev
a
a,
O. V.
Venidictova
b,
T. M.
Valova
b,
V. A.
Barachevsky
b,
L. M.
Khalilov
a and
U. M.
Dzhemilev
a
aInstitute of Petrochemistry and Catalysis, Russian Academy of Sciences, Russian Federation. E-mail: tuktarovar@gmail.com
bPhotochemistry Centre, Russian Academy of Sciences, Russian Federation
First published on 26th July 2016
Abstract
The 1,3-dipolar cycloaddition of azomethine ylides to fullerene C60 was utilized to perform the synthesis of spiropyran-containing photochromic pyrrolidinofullerenes. It was found that photochromism is observed only for the hybrid compound containing an NO2 group in the pyran moiety, whereas the pyrrolidinofullerenes with Cl or F atoms in the spiropyran moiety do not possess photochromism. The photochromic fullerene hybrid is characterized by lower light sensitivity and photodegradation parameters compared to the initial spiropyrans, which can be attributed to reabsorption of activating radiation.
Introduction
Synthesis of novel organic and organometallic photochromic molecules that undergo reversible photoisomerization is one of the major areas of focus in modern chemistry. Huge interest in such photochromic compounds is driven by the possibility of development of memory elements,1 optoelectronic devices,2 sensors3etc. based on them.Among the most popular and extensively studied photochromic molecules are diarylethenes, spiropyrans and spirooxazines, fulgides, as well as azobenzene. The isomeric forms of these compounds differ substantially in their physical and optical properties,4 which led to the appearance of a great number of investigations concerning their applications as dynamic materials.5 Of specific importance in this research area are studies devoted to the creation of nanomaterials with controlled properties, in particular, light sensitizing of carbon nanomolecules: fullerenes, nanotubes, and graphene.6 Investigations of covalent and molecular binding of such carbon clusters to dithienylethenes,7 spirooxazines8 and azobenzene9 have been reported, which made it possible to obtain conceptually new materials for various fields of science and technology.
One possible way to use such hybrid materials is design of various sensors. These devices employ the modification of the electronic properties of hybrid molecule. It was earlier shown,8a for example, that spiropyran attached to fullerene C60via covalent binding can exhibit allochroic properties in polar solvents and in acid or alkali media as well. In the present paper, we demonstrate that the electronic characteristics of hybrid molecules based on [60]fullerene and spiropyrane can also be tailored by light.
Considering the foregoing and also aiming to extend the class of photochromic carbon clusters and to study the influence of the fullerene cage and the photochromic moiety on the electronic properties and stability of the new hybrid molecule, we performed covalent binding of C60 to spiropyrans.
Result and discussion
The well known reaction of fullerene C60 with in situ generated azomethine ylides (Prato reaction),10 which makes it possible to obtain pyrrolidinofullerenes in preparative yields, was used as the basis for the development method.The experiments showed that the reaction between fullerene C60 and spiropyran 1, which contains an aldehyde and a nitro group in the molecule, in the presence of sarcosine leads to the formation of fulleropyrrolidine 4 in ∼60% yield (Scheme 1). Hybrid compounds 5 and 6 were obtained in a similar way.
Compounds 4–6 were isolated from the reaction mixture by column chromatography (SiO2). Elution with toluene recovered the unreacted C60, and after that, the use of pyridine as the eluent afforded the target adducts 4–6 with ∼100% purity. The structures of pure fulleropyrrolidines 4–6 were reliably determined by one- (1H and 13C) and two-dimensional (H–H COSY, HSQC, and HMBC) NMR spectroscopy and MALDI TOF/TOF mass spectrometry.
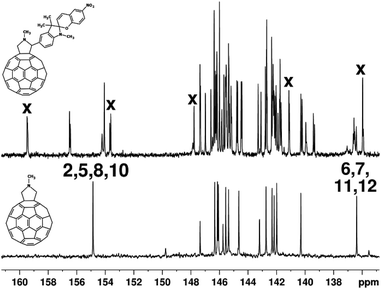
The pyrrolidine moiety of hybrid molecules 4–6 is responsible for 13C NMR signals at δC 70.11 (C5), 83.82 (C2), and 40.12 (N–CH3) correlated in the HSQC experiment with proton signals at δH 4.29 (HA–C5), 4.99 (HB–C5), 4.92 (H–C2), and 2.91 (N–CH3) ppm, respectively. Furthermore, according to HMBC data, five quaternary carbon atoms of the spiro-oxazine moiety incorporated in aromatic rings give rise to the signals at δ 135.96, 141.13, 147.77, 153.62, and 159.47, located in the same region as the signals characteristic of the sp2-hybridized carbon atoms of the fullerene cage (Fig. 1). Therefore, the 13C NMR spectrum of spiro-oxazine derivative 4 exhibits 48 signals of the sp2-hybridized carbon atoms of the fullerene cage, indicating the C1 point group of symmetry for this molecule.
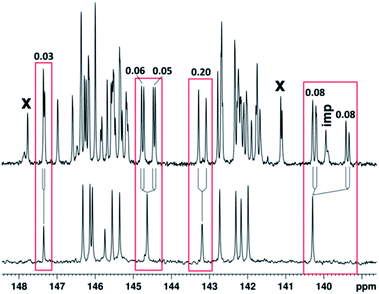
This signal splitting is likely to be caused by the effect of the chiral carbon atom of the pyrrolidine ring, which was observed in our previous studies for fullerene derivatives containing chiral centers in the attached moieties.11 No influence of the second chiral carbon atom involved into the spiropyran moiety was revealed because it locates by seven chemical bonds away from the fullerene core.12 According to quantum chemical calculations by the GIAO method at the X3LYP/6-31G level of theory,13 the high-frequency split signals at δ 156.45, 156.50, 154.06, and 154.23 refer to C2, C5, C8, and C10 atoms of the fullerene cage, respectively. It is noteworthy that the C2 and C5 atoms occurring in the sp2-hybridized state are linked to the sp3-hybridized C1 atom of the fullerene cage and, similarly, C8 and C10 atoms are linked to C9, thus forming so-called α-environment of C1 and C9. In turn, the β-environment for the same sp3 atoms is formed by C6, C7, C11, and C12 atoms, which are responsible for low-frequency signals at δ 136.41, 136.52, 136.57, and 136.62, respectively. This observation supports the previously formulated regularity, stating that the sp2 carbon atoms of the fullerene sphere producing the most low-frequency signals are linked to those responsible for high-frequency resonance signals.14 Simultaneously, the degree of influence of the chiral carbon atom located in the pyrrolidine ring of the attached moiety can be estimated from the diastereotopic splitting of the signals of carbon atoms located symmetrically relative to each other in the fullerene cage. This can be done by comparing the 13C NMR spectrum of spiro-oxazine adduct 4 with that of 2,5-unsubstituted pyrrolidinofullerene, which corresponds to the C2V point group of symmetry (Fig. 1 and 2).
The pair signals at δ 139.34 and 139.42, as well as 140.21 and 140.29 with 1C intensity in the spectrum of compound 4 merge to form a single signal of 4C intensity in the spectrum of 2,5-unsubstituted pyrrolidinofullerene devoid of the chiral center; this signal refers to four magnetically equivalent and symmetrically located C15, C18, C24, and C27 carbon atoms. The diastereotopic splitting Δδdias is calculated as the difference between the chemical shifts of the pair signals Δδdias(C15, C18) = 139.42–139.34 ppm = 0.08 ppm and Δδdias(C24, C27) = 140.29–140.21 ppm = 0.08 ppm. The diastereotopic splittings for other carbon atoms were determined in a similar way (Fig. 2).
The structures of the synthesized fulleropyrrolidines 4–6 were also determined with good reliability with MALDI TOF/TOF mass spectrometry, the spectra being characterized by intensive [M − 3H] molecular ion peaks with m/z 1096.1, 1085.1, and 1069.1 respectively.
The results of the studies of photochromic properties for compounds 4–6 are summarized in Table 1 (see ESI†).
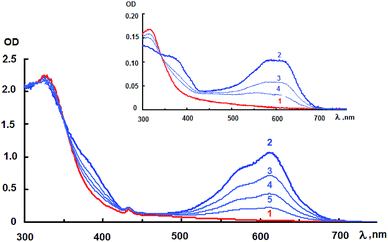
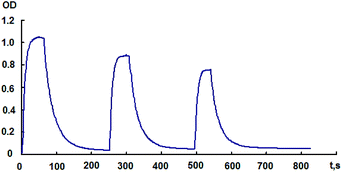
Fig. 3 (inset) shows the photoinduced spectral changes of the initial NO2-containing spiropyran 1 in toluene. It can be seen that the absorption spectrum of the photoinduced merocyanine form has two absorption maxima in the visible region at 580 and 620 nm. During the dark relaxation process, the intensity of photoinduced absorption bands gradually decreases. Similar photoinduced spectral changes are also observed for the hybrid compound 4 in toluene. In this case, the long-wavelength absorption maximum was more clear-cut and located at 610 nm.
The decline in the amplitude of variation of the absorption band intensity of the photoinduced merocyanine form of spiropyran in hybrid 4 also indicates that this compound is subject to photochromic transformations accompanied by a gradual photodegradation (Fig. 4). It should also be noted that no special study to establish influence of molecular oxygen on fatigue resistance and photodegradation of the photochromic compound 4 was carried out, so that it will be a purpose of the future works.
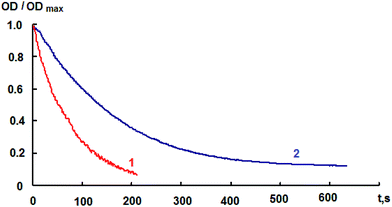
It can be concluded from comparison of the curves presented in Fig. 3 that there are no significant spectral distinctions between the nitro-substituted spiropyran 1 and its hybrid compound with fullerene 4, which is presumably indicative of weak interaction between the spiropyran and fullerene moieties. There is a difference in the light-sensitivity, which is determined by ΔODphotB/ODmaxA ratio (Table 1, ESI†). This ratio represents the photoinduced change in the optical density at the absorption maximum of the merocyanine form normalized to the maximum intensity of the absorption band of the initial spiran form, which absorbs the activating radiation. The light-sensitivity of hybrid compound 4 is lower in both toluene and chloroform compared to the initial spiropyran 1 (Table 1, ESI†). Substantial difference in the photodegradation of these compounds is observed (Fig. 5). Pyrrolidinofullerene 4 was found to be more resistant to irreversible phototransformations. The photodegradation of compound 4 decreases going from a toluene to a chloroform solution. The decrease in the light-sensitivity and enhancement of the photodegradation resistance of compound 4 can be attributed to the reabsorption of the activating radiation by the fullerene moiety, which is located in the absorption area of the photochromic fragment.
The influence of the fullerene moiety in the hybrid photochromic compound 4 is manifested as a decrease in the rate of spontaneous dark bleaching of the merocyanine form in both toluene and chloroform (Table 1, ESI†). This may serve as evidence for covalent bonding between the spiropyran molecule and the fullerene moiety.
Photochromic transformations of the initial spiropyran 1 and pyrrolidinofullerene 4 were also found for amorphous films (Fig. 4, Table 1 in ESI†).
Unfortunately, unlike photochromic compounds 2 and 3 pyrrolidinofullerenes 5 and 6 do not exhibit photochromic transformations upon either constant or pulsed photoexcitation. This is not related to quick spontaneous dark relaxation of the photoinduced form, because compound 4 is characterized by lower bleaching rate compared to the initial photochromic compound 1. Reabsorption of the activating light by the fullerene moiety may serve as a possible explanation, as in pyrrolidinofullerenes 5 and 6, the absorption bands of the spiran forms of the initial compounds 2 and 3 effectively overlap with the fullerene absorption spectrum (see ESI†). In the case of the initial spiropyran 1, only partial overlap of the spectra is observed for pyrrolidinofullerene 4, making it possible to witness photochromic transformations of the hybrid molecule 4. Another possible explanation for decreasing light-sensitivity of compound 4 and the lack of photochromism of pyrrolidinofullerenes 5 and 6 is the photo-induced electron transfer from the photochromic fragment to electron accepter fullerene moiety.
Conclusions
Thus, we accomplished for the first time the covalent binding of photochromic spiropyrans to fullerene C60 and thus obtained novel hybrid molecules, characterized by photochromism and substantial resistance to irreversible phototransformations, unlike the initial substrates. This opens up prospects for the application of such hybrid molecules in organic field effect transistors, solar energy converters, and memory elements.Acknowledgements
This work was financially supported by a grant of the Russian Science Foundation (No. 14-13-00296).Notes and references
-
(a) H. Bouas-Laurent and H. Dürr, Pure Appl. Chem., 2001, 73, 639 CrossRef CAS
; (b) E. Orgiu and P. Samori, Adv. Mater., 2014, 26, 1827 CrossRef CAS PubMed
.
- M. Tomasulo, I. Yildiz and F. M. Raymo, Inorg. Chim. Acta, 2007, 360, 938 CrossRef CAS
.
-
(a) K. Kimura, Coord. Chem. Rev., 1996, 148, 41 CrossRef CAS
; (b) I. Willner, Acc. Chem. Res., 1997, 30, 347 CrossRef CAS
; (c) L. Evans, G. E. Collins, R. E. Shaffer, V. Mechelet and J. D. Winkler, Anal. Chem., 1999, 71, 5322 CrossRef CAS PubMed
; (d) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS
; (e) V. A. Bren, Russ. Chem. Rev., 2001, 70, 1152 CrossRef
; (f) X. Xie, G. Mistlberger and E. Bakker, J. Am. Chem. Soc., 2012, 134, 16929 CrossRef CAS PubMed
; (g) M. Qin, Y. Huang, F. Li and Y. Song, J. Mater. Chem. C, 2015, 3, 9265 RSC
; (h) E. N. Shepelenko, Y. V. Revinskii, K. S. Tikhomirova, O. G. Karamov, A. D. Dubonosov, V. A. Bren and V. I. Minkin, Mendeleev Commun., 2016, 26, 193 CrossRef CAS
.
-
(a) V. I. Minkin, Chem. Rev., 2004, 104, 2751 CrossRef CAS PubMed
; (b) M. M. Krayushkin, A. M. Bogacheva, K. S. Levchenko, O. I. Kobeleva, T. M. Valova, V. A. Barachevskii, J.-L. Pozzo, M. I. Struchkova, P. S. Shmelin, M. A. Kalik, T. K. Baryshnikova and V. N. Charushin, Mendeleev Commun., 2013, 23, 78 CrossRef CAS
.
- R. Klajn, Chem. Soc. Rev., 2014, 43, 148 RSC
.
- X. Zhang, L. Hou and P. Samori, Nat. Commun., 2016, 7, 11118 CrossRef CAS PubMed
.
-
(a) P. A. Liddell, G. Kodis, A. L. Moore, T. A. Moore and D. Gust, J. Am. Chem. Soc., 2002, 124, 7668 CrossRef CAS PubMed
; (b) H.-Y. Hu, M.-Z. Zhu, Z.-P. Zhang, G.-T. Wen, Y. Fu and Q.-X. Guo, Chin. J. Chem. Phys., 2006, 19, 433 CrossRef CAS
; (c) A. C. Whalley, M. L. Steigerwald, X. Guo and C. Nuckolls, J. Am. Chem. Soc., 2007, 129, 12590 CrossRef CAS PubMed
; (d) C. Motta, M. I. Trioni, G. P. Brivio and K. L. Sebastian, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 113408 CrossRef
; (e) S. Castellanos, A. A. Vieira, B. M. Illescas, V. Sacchetti, C. Schubert, J. Moreno, D. M. Guldi, S. Hecht and N. Martin, Angew. Chem., Int. Ed., 2013, 52, 13985 CrossRef CAS PubMed
; (f) A. R. Tuktarov, A. A. Khuzin, A. R. Akhmetov, V. A. Barachevsky, O. V. Venidiktova and U. M. Dzhemilev, Tetrahedron Lett., 2015, 56, 7154 CrossRef CAS
; (g) A. R. Tuktarov, A. A. Khuzin, A. R. Akhmetov, L. M. Khalilov, A. R. Tulyabaev, V. A. Barachevsky, O. V. Venidiktova and U. M. Dzhemilev, Mendeleev Commun., 2016, 26, 143 CrossRef CAS
.
-
(a) J.-H. Xu, Y.-L. Li and D.-B. Zhu, Synth. Commun., 2002, 32, 1647 CrossRef CAS
; (b) X. Guo, L. Huang, S. O'Brien, P. Kim and C. Nuckollls, J. Am. Chem. Soc., 2005, 127, 15045 CrossRef CAS PubMed
; (c) E. Del Canto, K. Flavin, M. Natali, T. Perova and S. Giordani, Carbon, 2010, 48, 2815 CrossRef CAS
; (d) P. Joo, B. J. Kim, E. K. Jeon, J. H. Cho and B.-S. Kim, Chem. Commun., 2012, 48, 10978 RSC
; (e) A.-R. Jang, E. K. Jeon, D. Kang, G. Kim, B.-S. Kim, D. J. Kang and H. S. Shin, ACS Nano, 2012, 6, 9207 CrossRef CAS PubMed
; (f) J. Frey, G. Kodis, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, J. Phys. Chem. A, 2013, 117, 607 CrossRef CAS PubMed
; (g) A.-A. Nahain, J.-E. Lee, J. H. Jeong and S. Y. Park, Biomacromolecules, 2013, 14, 4082 CrossRef CAS PubMed
; (h) Y. Li, Y. Duan, J. Zheng, J. Li, W. Zhao, S. Yang and R. Yang, Anal. Chem., 2013, 85, 11456 CrossRef CAS PubMed
; (i) S. M. Sharker, C. J. Jeong, S. M. Kim, J.-E. Lee, J. H. Jeong, I. In, H. Lee and S. Y. Park, Chem.–Asian J., 2014, 9, 2921 CrossRef CAS PubMed
; (j) A. Perry, S. J. Green, D. W. Horsell, S. M. Hornett and M. E. Wood, Tetrahedron, 2015, 71, 6776 CrossRef CAS
.
-
(a) K. Oh-ishi, J. Okamura, T. Ishi-i, M. Sano and S. Shinkai, Langmuir, 1999, 15, 2224 CrossRef CAS
; (b) K.-Y. Kay, K.-J. Han, Y.-J. Yua and Y. D. Park, Tetrahedron Lett., 2002, 43, 5053 CrossRef CAS
; (c) Y. Yang, X. Wang, L. Liu, X. Xie, Z. Yang, R. K. Y. Li and Y.-W. Mai, J. Phys. Chem. C, 2007, 111, 11231 CrossRef CAS
; (d) J. M. Simmons, I. In, V. E. Campbell, T. J. Mark, F. Leonard, P. Gopalan and M. A. Eriksson, Phys. Rev. Lett., 2007, 98, 086802 CrossRef CAS PubMed
; (e) Y. Feng, X. Zhang, X. Ding and W. Feng, Carbon, 2010, 48, 3091 CrossRef CAS
; (f) Y. Cao, S. Dong, S. Liu, Z. Liu and X. Guo, Angew. Chem., Int. Ed., 2013, 52, 3906 CrossRef CAS PubMed
; (g) K. Yuan, Y.-J. Guo and X. Zhao, Phys. Chem. Chem. Phys., 2014, 16, 27053 RSC
; (h) S. Ma, H. Ting, L. Zhang, Y. Ma, L. Zheng, L. Xiao and Z. Chen, Org. Electron., 2015, 23, 1 CrossRef CAS
; (i) W. Luo, Y. Feng, C. Cao, M. Li, E. Liu, S. Li, C. Qin, W. Hu and W. Feng, J. Mater. Chem. A, 2015, 3, 11787 RSC
; (j) W. Luo, Y. Feng, C. Qin, M. Li, S. Li, C. Cao, P. Long, E. Liu, W. Hu, K. Yoshino and W. Feng, Nanoscale, 2015, 7, 16214 RSC
.
- M. Maggini, G. Scorrano and M. Prato, J. Am. Chem. Soc., 1993, 115, 9798 CrossRef CAS
.
-
(a) L. M. Khalilov, A. R. Tulyabaev and A. R. Tuktarov, Magn. Reson. Chem., 2011, 49, 768 CrossRef CAS PubMed
; (b) A. R. Tulyabaev, A. R. Tuktarov and L. M. Khalilov, Magn. Reson. Chem., 2014, 52, 3 CrossRef CAS PubMed
.
- J. I. Kroschwitz, M. Winokur, H. J. Reich and J. D. Roberts, J. Am. Chem. Soc., 1969, 91, 5927 CrossRef CAS
.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision E.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed
.
-
(a) M. S. Meier, H. P. Spielmann, R. G. Bergosh and R. C. Haddon, J. Am. Chem. Soc., 2002, 124, 8090 CrossRef CAS PubMed
; (b) M. S. Meier, H. P. Spielmann, R. G. Bergosh and M. C. Tetreau, J. Org. Chem., 2003, 68, 7867 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18073f |
| This journal is © The Royal Society of Chemistry 2016 |