Charge-assisted intermolecular hydrogen bond formed in coamorphous system is important to relieve the pH-dependent solubility behavior of lurasidone hydrochloride†
Shuai Qian‡
a,
Zhen Li‡b,
Weili Henga,
Shujun Liangb,
Di Maa,
Yuan Gaoa,
Jianjun Zhang*b and
Yuanfeng Wei*a
aSchool of Traditional Chinese Medicine, China Pharmaceutical University, Nanjing 210009, P. R. China. E-mail: weiyuanfengyuer@yeah.net; Tel: +86 152 5157 6256
bSchool of Pharmacy, China Pharmaceutical University, Nanjing 210009, P. R. China. E-mail: myamicute@163.com; Fax: +86 25 83379418; Tel: +86 25 83379418
First published on 24th October 2016
Abstract
The aim of this study was to enhance the dissolution properties of two BSC II drugs (lurasidone hydrochloride (LH) and repaglinide (REP)) by a coamorphization technique. Coamorphous LH–REP systems (CMs) were prepared by solvent evaporation and characterized by DSC, XRPD, FTIR, Raman spectroscopy, and Ss 13C NMR. The solubilities and intrinsic and supersaturated dissolution profiles, as well as the physical stability, of CMs were compared with the properties of amorphous LH, amorphous REP and their physical mixtures. The single Tg observed in DSC and the disappearance of crystallinity in XRPD indicated the formation of CMs. Principal component analysis of FTIR in combination with Raman spectroscopy and Ss 13C NMR suggested the absence of intermolecular interactions in CMs. In comparison to the pure amorphous forms and their physical mixtures, CMs displayed greatly improved physical stability. In addition, in contrast to the pure amorphous forms, which exhibited temporary enhancements in dissolution, both drugs in CMs exhibited persistent increases in IDRs and supersaturated dissolution, as well as significantly improved solubilities. The persistent pH-dependent solubility behavior of LH in CMs suggested that intermolecular interaction with the N+–H group in the LH structure was probably essential for improving the pH-dependent solubility profile of LH, but was not critical for achieving supersaturated dissolution and preventing the conversion of coamorphous components.
1. Introduction
The low aqueous solubility of active pharmaceutical ingredients has always been considered as one of the most challenging properties associated with various formulation-related performance issues. With more than 75% of drug candidates regarded as poorly soluble in water and belonging to BCS classes II and IV,1,2 improving the bioavailability of these drugs by enhancing their solubility/dissolution has raised widespread concern in the pharmaceutical industry. Amorphous systems with a lack of long-range order in their molecular packing in a high-energy state have been extensively investigated as a promising approach for enhancing the solubility/dissolution and hence the bioavailability/drug efficacy of BCS class II and IV drugs.3 However, the inherently poor physical stability of amorphous solids (which have a tendency to structural relaxation and crystallization during manufacture, storage and dissolution) has greatly restricted their employment in the development of drug products.4,5In order to improve the physical stability of amorphous drugs under storage and dissolution conditions, polymer-based and mesoporous silica-based amorphous solid dispersions (ASDs) have been employed and extensively studied.6,7 Most polymeric carriers have a high glass transition temperature (Tg) and thus increase the Tg of drugs in ASDs in comparison to that of their pure amorphous forms. In addition, intermolecular interactions between drugs and functional groups of polymers could further reduce the molecular mobility of drugs and their ability to nucleate and crystallize, and hence stabilize the amorphous state of ASDs.4,8 In mesoporous silica-based ASDs, amorphous drugs adsorbed on the surface of silica particles (a stable hydrophilic matrix with a nanoporous structure) are stabilized via intermolecular interactions between the drugs and functional groups of the silica matrix.9 However, the limited drug loading capacity of both the above mentioned ASDs would lead to a large bulk volume/mass of the final dosage forms when drugs are poorly miscible with polymers and the silica matrix.10,11 Furthermore, the hygroscopic nature of many polymeric carriers would result in the absorption of moisture as a plasticizer and facilitate the recrystallization of the incorporated amorphous drugs.12
Coamorphous drug delivery systems, which are a new glass solution type of ASDs characterized by the combination of two or more low-molecular-weight components that form a homogeneous amorphous single-phase system, have recently gained increasing interest in the pharmaceutical field owing to their potential to enhance the dissolution and stability of BCS class II and IV drugs in the amorphous state,13 as well as overcoming shortcomings associated with conventional ASDs. So far, two types of coamorphous system have been proposed, namely, drug–drug combinations and drug–excipient mixtures.14 In the first type, two pharmacologically relevant drugs (same type or different types of drug in pharmacodynamic effect) with synergistic effects that are intended for multi-drug or multi-target therapies are usually combined. It has been discovered that coamorphous drug mixtures of naproxen and indomethacin,15 simvastatin and glipizide,16 indomethacin and ranitidine hydrochloride,17 and cimetidine and diflunisal18 demonstrated significant increases in their solubility and/or dissolution rate in comparison to their individual amorphous forms. In addition, coamorphization of talinolol (a p-gp substrate) with the flavonoid naringin (a p-gp inhibitor), which is a dual-track strategy that simultaneously enhances solubility/dissolution and permeability, gave rise to a 5.4-fold increase in oral bioavailability in comparison to that of talinolol alone.19 In drug–excipient coamorphous systems, a number of small-molecule excipients, in particular with hydrogen donors and/or receptors (saccharin,20,21 citric acid,22,23 and amino acids,24,25 etc.), have been employed as coamorphous coformers of poorly soluble drugs.
Lurasidone hydrochloride (LH, Fig. 1a), which is a first-line atypical antipsychotic agent for the treatment of schizophrenia and bipolar disorder,26 is a BCS class II drug with poor oral bioavailability (9–19%) due to its low dissolution.27,28 Pharmacoepidemiological studies have shown that diabetes is commonly seen in 20% of patients with schizophrenia. It is more prevalent than in the general population and contributes to the increased morbidity and shortened lifespan seen in this population.29,30 In addition, atypical antipsychotics (e.g., LH, clozapine, and olanzapine, etc.) have been associated with hyperglycemia, which may alter blood glucose control.31,32 Thus, antidiabetic agents (e.g., metformin, repaglinide (REP), and glipizide) are commonly combined with antipsychotics for these patients under a physician's supervision.33–35
REP (Fig. 1b), which is an antidiabetic drug in the class of meglitinides, is clinically used to manage type 2 diabetes mellitus by stimulating the secretion of insulin by the pancreas.36 As a BCS class II drug, REP has a quite low aqueous solubility. In our previous study, REP was coamorphized with saccharin by a solvent evaporation method, which led to significant increases in the solubility (∼18-fold in pure water) and dissolution rate of REP in various aqueous media in comparison to those of crystalline REP.20 In addition, saccharin was also employed as a coamorphous coformer of LH. In addition to greatly improving the solubility and dissolution rate (∼5-fold) of LH, the LH–saccharin coamorphous system that was prepared displayed superior physical stability in comparison to that of amorphous LH during dissolution and under conditions of long-term storage in our previous findings.21 Moreover, in contrast to pure crystalline and amorphous LH, which exhibited pH-dependent solubility behavior, coamorphous LH–saccharin demonstrated a pH-independent solubility profile in the pH range of 2–5.5 (which would result in a decrease in individual variations in dissolution in gastric juice with different pH values under fasting/fed conditions or physiological variations), which was ascribed to a coamorphization effect and the formation of charge-assisted hydrogen bonds between the N+–H groups of LH and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of saccharin in such coamorphous systems.
O groups of saccharin in such coamorphous systems.
In the current study, coamorphous LH–REP systems in various molar ratios were prepared and characterized with respect to their thermal and dissolution properties, possible intermolecular interactions, and physical stability. In addition, we also assessed whether the coamorphization of LH with REP would also improve the pH-dependent solubility behavior of LH, as was previously reported for coamorphous LH–saccharin. This study will determine the effect of the presence or absence of intermolecular interactions in coamorphous systems on various physicochemical properties of LH.
2. Materials and methods
2.1. Materials
Lurasidone hydrochloride (LH, Mw = 529.1 g mol−1) was synthesized and gifted by Changzhou Yinsheng Pharmaceutical Co., Ltd. (Changzhou, China). Lurasidone (L) in its base form was prepared by stoichiometric titration with sodium hydroxide. Repaglinide (REP, Mw = 452.6 g mol−1) was obtained from Zhejiang Hisoar Pharmaceutical Co., Ltd. (Taizhou, China). Methanol of HPLC grade was purchased from E. Merck (Darmstadt, Germany). All other chemical reagents were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).2.2. Methods
Coamorphous LH–REP systems in molar ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (CMs (2
2 (CMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (1
1), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (1
1) and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) were prepared by the same method as was described above. The amorphous LH, amorphous REP and CMs that were obtained were sieved through a 100 mesh (about 150 μm) screen and stored in a vacuum desiccator over anhydrous calcium chloride at 4 °C for further characterization.
2)) were prepared by the same method as was described above. The amorphous LH, amorphous REP and CMs that were obtained were sieved through a 100 mesh (about 150 μm) screen and stored in a vacuum desiccator over anhydrous calcium chloride at 4 °C for further characterization.
The Gordon–Taylor equation was used to calculate the theoretical value of Tg, and the accuracy of this equation has been widely verified:37,38
 | (1) |
 | (2) |
Raman spectra of samples were recorded at room temperature using a DXR laser micro-Raman spectrometer (Thermo Fisher Scientific Inc., USA) with a 780 nm excitation laser. The spectra were recorded over the range of 200–3200 cm−1 at a spectral resolution of 2 cm−1. The spectral data were analyzed using OMNIC software (Thermo Scientific™).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) (PM (1
1 molar ratio) (PM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)), a physical mixture of amorphous LH and amorphous REP (1
1)), a physical mixture of amorphous LH and amorphous REP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) (APM (1
1 molar ratio) (APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)), and CM (1
1)), and CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and 10 mL of 0.05 M phosphate buffer solutions (PBS, pH 2.0, 3.0, 3.8, 4.5, 5.0, 5.5, 6.0 and 6.8) were placed in a water bath and stirred with a magnetic bar. After being stirred for 24 h at 25 °C, the slurry was filtered through a 0.22 μm nylon filter (Millipore, Bedford, MA) (24 hours was enough to reach solubility equilibrium; data not shown). The concentrations of LH and/or REP in the obtained filtrates were determined by subsequent HPLC/UV analysis after appropriate dilution. Each experiment was repeated in triplicate. LH and REP were simultaneously baseline-separated with retention times of 8.5 min and 5.0 min, respectively, by an Inertsil ODS-SP column (150 × 4.6 mm, 5 μm) in a Shimadzu LC-2010AHT HPLC system (Shimadzu Corporation, Kyoto, Japan). The mobile phase, which consisted of 70% acetonitrile and 30% aqueous buffer (containing 0.05% triethylamine and 0.05% acetic acid), was run at 1.0 mL min−1 with a detection wavelength of 230 nm. The column temperature was set at 30 °C. Within the concentration ranges of 8.0–80.3 μg mL−1 and 8.6–86.1 μg mL−1 for LH and REP, respectively, good linearity (r2 > 0.998) was achieved. The relative standard deviations (% RSD) of the intra-day and inter-day precision for each analyte at three quality control concentrations within the linear range were below 5%, and the accuracy was within the range of 95–105%. The limit of quantification (LOQ) for LH and REP was found to be 4.9 ng mL−1 and 5.5 ng mL−1, respectively. LH and REP solutions prepared using the mobile phase were found to be stable in an autosampler under ambient conditions for at least 24 hours.
1)) and 10 mL of 0.05 M phosphate buffer solutions (PBS, pH 2.0, 3.0, 3.8, 4.5, 5.0, 5.5, 6.0 and 6.8) were placed in a water bath and stirred with a magnetic bar. After being stirred for 24 h at 25 °C, the slurry was filtered through a 0.22 μm nylon filter (Millipore, Bedford, MA) (24 hours was enough to reach solubility equilibrium; data not shown). The concentrations of LH and/or REP in the obtained filtrates were determined by subsequent HPLC/UV analysis after appropriate dilution. Each experiment was repeated in triplicate. LH and REP were simultaneously baseline-separated with retention times of 8.5 min and 5.0 min, respectively, by an Inertsil ODS-SP column (150 × 4.6 mm, 5 μm) in a Shimadzu LC-2010AHT HPLC system (Shimadzu Corporation, Kyoto, Japan). The mobile phase, which consisted of 70% acetonitrile and 30% aqueous buffer (containing 0.05% triethylamine and 0.05% acetic acid), was run at 1.0 mL min−1 with a detection wavelength of 230 nm. The column temperature was set at 30 °C. Within the concentration ranges of 8.0–80.3 μg mL−1 and 8.6–86.1 μg mL−1 for LH and REP, respectively, good linearity (r2 > 0.998) was achieved. The relative standard deviations (% RSD) of the intra-day and inter-day precision for each analyte at three quality control concentrations within the linear range were below 5%, and the accuracy was within the range of 95–105%. The limit of quantification (LOQ) for LH and REP was found to be 4.9 ng mL−1 and 5.5 ng mL−1, respectively. LH and REP solutions prepared using the mobile phase were found to be stable in an autosampler under ambient conditions for at least 24 hours.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or CM (1
1) or CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) was compressed at a pressure of 115.2 MPa for 10 s using a hydraulic press (Jintan Ruiding Machinery Co., Ltd., Changzhou, China). The resulting discs, which had a surface area of 0.5024 cm2, were inserted into a PTFE intrinsic dissolution sample holder to obtain a flat surface; eventually, only one surface of each tablet was exposed to the dissolution medium.
1)) was compressed at a pressure of 115.2 MPa for 10 s using a hydraulic press (Jintan Ruiding Machinery Co., Ltd., Changzhou, China). The resulting discs, which had a surface area of 0.5024 cm2, were inserted into a PTFE intrinsic dissolution sample holder to obtain a flat surface; eventually, only one surface of each tablet was exposed to the dissolution medium.A USP II dissolution apparatus was employed in studies of intrinsic dissolution. Dissolution tests (six replicates) were performed in 900 mL of 0.2 M PBS (pH 3.8) at 37 °C with a paddle rotation speed of 50 rpm. Three milliliter aliquots were withdrawn at predetermined time points (5, 10, 15, 20, 30, 40, 60 and 90 min) and analyzed by the abovementioned HPLC/UV method. To calculate the IDR of LH and REP, the cumulative amount dissolved per surface area of the tablet was plotted against time. The slope of the linear region (r2 ≥ 0.95) was taken to be the intrinsic dissolution rate.39
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (APMs), or CMs (2
2) (APMs), or CMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) equivalent to 500 mg LH) to the dissolution medium, 2 mL samples were withdrawn and filtered at predetermined time points (0.5, 1, 2, 4, 7, 10 and 24 h). The concentrations of LH and REP were simultaneously determined by the HPLC/UV method described above.
2) equivalent to 500 mg LH) to the dissolution medium, 2 mL samples were withdrawn and filtered at predetermined time points (0.5, 1, 2, 4, 7, 10 and 24 h). The concentrations of LH and REP were simultaneously determined by the HPLC/UV method described above.2.2.9.1. Physical stability. Amorphous LH, amorphous REP, APMs and CMs were exposed in a constant temperature/humidity chamber (Shanghai Boxun Industry & Commerce Co., Ltd., Shanghai, China) at 25 °C and 60% RH (long-term storage conditions). Samples were collected at predetermined times to investigate the physical stability of the amorphous materials by XRPD and polarized light microscopy (Leica, Wetzlar, Germany) for up to 90 days.
2.2.9.2. Chemical stability. The chemical stability of coamorphous LH–REP was studied immediately after its preparation and after its storage under different conditions for up to 90 days (i.e., 25 °C and 60% RH (long-term storage conditions), 40 °C and 60 °C in an oven). The experiment was conducted by dissolving a known amount of the sample in a mobile phase and analyzing the sample by HPLC using a photodiode array detector (PDA), with a slight change in the ratio of the mobile phase (65% acetonitrile and 35% aqueous buffer) from the above mentioned HPLC conditions (Section 2.2.6.). Extra peaks that appeared in the chromatogram were defined as impurities, and the content of each impurity was calculated by dividing its peak area by the area of the principal peak in the chromatogram obtained using a 0.1% reference solution.
3. Results and discussion
3.1. DSC
The DSC thermograms of crystalline LH, crystalline REP, amorphous LH, amorphous REP, PM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), APM (1
1), APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and CM (1
1), and CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are shown in Fig. 2. In contrast to the two crystalline solids (Fig. 2a and b), amorphous LH and amorphous REP (Fig. 2c and d) underwent glass transition events at 67.43 °C and 53.35 °C and exhibited recrystallization exothermic peaks at 174.94 °C and 118.92 °C, respectively, which agreed with their reported values.21,40 PM (1
1) are shown in Fig. 2. In contrast to the two crystalline solids (Fig. 2a and b), amorphous LH and amorphous REP (Fig. 2c and d) underwent glass transition events at 67.43 °C and 53.35 °C and exhibited recrystallization exothermic peaks at 174.94 °C and 118.92 °C, respectively, which agreed with their reported values.21,40 PM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. 2e) displayed a melting point at 133.1 °C, which may belong to REP (1.1 °C lower than that of pure crystalline REP (Fig. 2b), owing to impurity-induced depression of the melting point41), followed by degradation peaks of LH at around 246.5 °C. In the case of APM (1
1) (Fig. 2e) displayed a melting point at 133.1 °C, which may belong to REP (1.1 °C lower than that of pure crystalline REP (Fig. 2b), owing to impurity-induced depression of the melting point41), followed by degradation peaks of LH at around 246.5 °C. In the case of APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. 2f), two separate values of Tg belonging to LH and REP were observed at 63.48 °C and 53.56 °C, respectively. Such a phenomenon, which has also been observed for an amorphous mixture of simvastatin and glipizide, was ascribed to the presence of two separate phases in the APM system.16 On the other hand, only a single value of Tg at 58.73 °C was observed for CM (1
1) (Fig. 2f), two separate values of Tg belonging to LH and REP were observed at 63.48 °C and 53.56 °C, respectively. Such a phenomenon, which has also been observed for an amorphous mixture of simvastatin and glipizide, was ascribed to the presence of two separate phases in the APM system.16 On the other hand, only a single value of Tg at 58.73 °C was observed for CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. 2g), which indicated the formation of a single-phase coamorphous system of LH and REP. In comparison to CM (1
1) (Fig. 2g), which indicated the formation of a single-phase coamorphous system of LH and REP. In comparison to CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), APM (1
1), APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited a slightly lower recrystallization point (167.04 °C vs. 162.56 °C), which suggested that APM was easier to recrystallize than CM. Such a phenomenon might be attributed to a steric effect in the CM system that reduced the molecular mobility of the components.42
1) exhibited a slightly lower recrystallization point (167.04 °C vs. 162.56 °C), which suggested that APM was easier to recrystallize than CM. Such a phenomenon might be attributed to a steric effect in the CM system that reduced the molecular mobility of the components.42
In addition to CMs in molar ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, mixtures in two more ratios (1.5
2, mixtures in two more ratios (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) were also fabricated (DSC curves and XRPD patterns not shown) to fit the Gordon–Taylor equation (eqn (1)). The experimental and calculated values of Tg of the studied coamorphous systems are summarized in Table 1. The results showed that the higher the concentration of LH in the coamorphous system, the higher was the Tg obtained, which was consistent with previously reported findings.16,43,44 The Gordon–Taylor equation assumes that there are no intermolecular interactions in the binary system. In general, a positive deviation of the experimental value of Tg from the theoretical value would suggest a greater strength of the interaction between the two components than that existing in the individual components,45,46 whereas a negative deviation would indicate an increase in free volume or a decrease in the amount and strength of hydrogen bonding or π–π stacking owing to mixing.16 For our coamorphous LH–REP systems, no statistically significant deviation of the experimental Tg values from the theoretically calculated values was observed (p = 0.449, i.e., >0.05), which suggests the absence of any kind of intermolecular interaction between the two components, which was supported by the following FTIR, Raman spectroscopy and Ss 13C NMR characterizations.
1.5) were also fabricated (DSC curves and XRPD patterns not shown) to fit the Gordon–Taylor equation (eqn (1)). The experimental and calculated values of Tg of the studied coamorphous systems are summarized in Table 1. The results showed that the higher the concentration of LH in the coamorphous system, the higher was the Tg obtained, which was consistent with previously reported findings.16,43,44 The Gordon–Taylor equation assumes that there are no intermolecular interactions in the binary system. In general, a positive deviation of the experimental value of Tg from the theoretical value would suggest a greater strength of the interaction between the two components than that existing in the individual components,45,46 whereas a negative deviation would indicate an increase in free volume or a decrease in the amount and strength of hydrogen bonding or π–π stacking owing to mixing.16 For our coamorphous LH–REP systems, no statistically significant deviation of the experimental Tg values from the theoretically calculated values was observed (p = 0.449, i.e., >0.05), which suggests the absence of any kind of intermolecular interaction between the two components, which was supported by the following FTIR, Raman spectroscopy and Ss 13C NMR characterizations.
| Sample | Theoretical Tg (°C) | Experimental Tg (°C) | ΔTg/°C |
|---|---|---|---|
| Amorphous LH | — | 67.4 | — |
| Amorphous REP | — | 53.4 | — |
CM (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
62.1 | 60.8 | −1.3 |
CM (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
61.2 | 59.6 | −1.6 |
CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
59.8 | 58.7 | −1.1 |
CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) 1.5) |
58.5 | 58.7 | 0.2 |
CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
57.5 | 57.2 | −0.3 |
3.2. XRPD
The XRPD patterns of crystalline LH, crystalline REP, amorphous LH, amorphous REP, and CMs are shown in Fig. 3. Crystalline LH and crystalline REP (Fig. 3a and b) displayed their characteristic diffraction peaks, whereas their evaporation products exhibited halo patterns, which suggested the formation of amorphous LH and amorphous REP (Fig. 3c and d). In addition, the vacuum evaporation products of LH and REP in three molar ratios (Fig. 3e–g) also exhibited typical halo patterns in their diffractograms owing to the absence of crystallinity, which indicated the formation of coamorphous LH–REP systems. | ||
Fig. 3 XRPD patterns of crystalline LH (a), crystalline REP (b), amorphous LH (c), amorphous REP (d), CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (e), CM (1 1) (e), CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (f) and CM (2 2) (f) and CM (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (g). 1) (g). | ||
3.3. FTIR and Raman spectroscopy
FTIR measurements were carried out to investigate possible intermolecular interactions between the two components of coamorphous LH–REP systems. The FTIR spectra of the studied samples (crystalline LH, crystalline REP, amorphous LH, amorphous REP, PMs, APMs and CMs) are shown in Fig. 4. In comparison to the vibrational spectrum of crystalline REP (Fig. 4b), amorphous REP (Fig. 4d) displayed significant broadenings and shifts in its characteristic absorption peaks from 1636.4 to 1646.1 cm−1 (amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations), 1687.1 to 1724.6 cm−1 (C
O vibrations), 1687.1 to 1724.6 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations of carboxylic acid groups) and 3306.3 to 3293.1 cm−1 (amide N–H stretching vibrations), which agreed with those in a previous report.40 Changes in the positions and shapes of peaks after the amorphization of REP (Fig. 4d), as well as that of LH (Fig. 4c), might be due to disruption of the structured crystal lattice into an amorphous state with a lack of long-range order and rearrangements in molecular conformation.47 In addition, the relatively large shift (+37.5 wavenumbers) in the peak due to carboxylic C
O vibrations of carboxylic acid groups) and 3306.3 to 3293.1 cm−1 (amide N–H stretching vibrations), which agreed with those in a previous report.40 Changes in the positions and shapes of peaks after the amorphization of REP (Fig. 4d), as well as that of LH (Fig. 4c), might be due to disruption of the structured crystal lattice into an amorphous state with a lack of long-range order and rearrangements in molecular conformation.47 In addition, the relatively large shift (+37.5 wavenumbers) in the peak due to carboxylic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations after amorphization suggested the deformation of the hydrogen bond network in intramolecular carboxylic acid groups of crystalline REP, which might be due to a possible molecular rearrangement during amorphization.15
O vibrations after amorphization suggested the deformation of the hydrogen bond network in intramolecular carboxylic acid groups of crystalline REP, which might be due to a possible molecular rearrangement during amorphization.15
The FTIR spectra of the prepared PMs and APMs are compared with those of CMs shown in Fig. 4e–m. PMs and APMs displayed overlapping FTIR absorption peaks of LH and REP in their corresponding states. In comparison to APMs, the absorptions due to stretching vibrations of the most characteristic groups (in particular, the functional groups most likely to participate in hydrogen bonding between the two amorphous drugs, i.e., N+–H (2441.5 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1692.8 cm−1) in LH and N–H (3292.3 cm−1) and C
O (1692.8 cm−1) in LH and N–H (3292.3 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1724 and 1646 cm−1) in REP) in the coamorphous systems only exhibited slight differences with minor hypsochromic or bathochromic shifts, which might be due to the higher degree of dispersion after the formation of the coamorphous systems. No other significant shift was observed between APMs and CMs, which implied that no significant intermolecular interaction existed between LH and REP in the coamorphous systems.
O (1724 and 1646 cm−1) in REP) in the coamorphous systems only exhibited slight differences with minor hypsochromic or bathochromic shifts, which might be due to the higher degree of dispersion after the formation of the coamorphous systems. No other significant shift was observed between APMs and CMs, which implied that no significant intermolecular interaction existed between LH and REP in the coamorphous systems.
Owing to the complexity of the spectra and minor differences between APMs and CMs in their FTIR spectra, PCA analysis, which extracts the full original information from spectra, was carried out to confirm the above mentioned speculations by visual inspection of the spectra.48 As was discussed above, only minor differences in FTIR peak position and intensity were observed between CMs and APMs, whereas significant changes in peak width and position were detected between CMs and PMs. Such changes would be ascribed to two factors, namely, the change in composition and the change in the molecular environments of the individual drugs.16 In PCA analysis, the peak position and corresponding intensity of all the observed FTIR peaks were plotted on the x and y axes, respectively. Dimensionality reduction is performed in PCA to map the data linearly in a lower-dimensional space. In such a way, the variance of the data in the lower-dimensional representation is maximized. In the current study, there are two principal components, namely, PC-1, which arises from the different compositions, and PC-2, which arises from the difference between crystalline and amorphous states, with a cumulative contribution ratio that reaches more than 96.9% (screen plot not shown). The score plot of the PCA analyses for FTIR is shown in Fig. 5. PC-1 was able to describe variations in chemical composition, because crystalline LH and REP (or amorphous LH and REP) displayed quite different PC-1 scores in two separate quadrants. On the other hand, PC-2 distinguished between crystalline and amorphous solid states, because crystalline LH and crystalline REP are obviously separated from their amorphous counterparts by the x axis in the score plot. The distance between crystalline LH and amorphous LH is obviously much greater than that between crystalline REP and amorphous REP, which agrees with the greater changes (such as shifts and broadenings of peaks, in particular the absorption due to N+–H vibrations at 2258 cm−1) in the FTIR spectrum of LH after amorphization than in that of REP (Fig. 4a–d). In addition, the fact that the distance between crystalline LH and crystalline REP is greater than that between amorphous LH and amorphous REP might be due to a peak broadening effect caused by amorphization, which reduced the differences in FTIR spectra among the chemical compositions. PMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) displayed a negative correlation with crystalline REP but a positive correlation with crystalline LH, which indicated that the FTIR spectra of PMs should simply comprise the superimposed FTIR spectra of the individual drugs, which agreed with the abovementioned experimental FTIR results (Fig. 4). In the score plot, APMs (2
2) displayed a negative correlation with crystalline REP but a positive correlation with crystalline LH, which indicated that the FTIR spectra of PMs should simply comprise the superimposed FTIR spectra of the individual drugs, which agreed with the abovementioned experimental FTIR results (Fig. 4). In the score plot, APMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and CMs (2
2) and CMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were closely gathered and clearly separated from PMs (2
2) were closely gathered and clearly separated from PMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), which suggested similarity within these groups.
2), which suggested similarity within these groups.
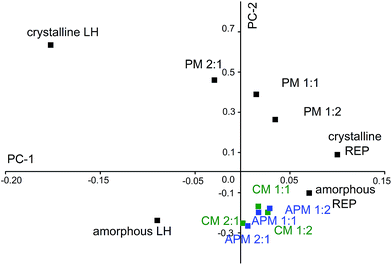 | ||
Fig. 5 Score plot of crystalline LH and REP, amorphous LH and REP, PMs, APMs and CMs in molar ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 from PCA analysis of their FTIR spectra. 2 from PCA analysis of their FTIR spectra. | ||
Infrared spectrometry is applicable for studying the asymmetric vibrations of polar groups, whereas Raman spectrometry is suitable for studying the symmetric vibrations of non-polar groups and the molecular skeleton. A combination of these two complementary spectral techniques has always been employed to analyze complex structural information.49 In order to further investigate possible changes in the vibrations of non-polar groups and the skeleton of LH and REP after coamorphization, Raman spectra of APMs and CMs were recorded and are shown in Fig. 6. In comparison to APMs, CMs exhibited almost the same peak positions with changes in intensity alone, which further indicated that there were no intermolecular interactions, but some differences in the molecular conformation and/or environment, after coamorphization.
3.4. Ss 13C NMR
Solid-state NMR has now become a routine technique for providing information on local molecular structure for a wide range of industrial applications or research purposes.50 It does not rely on long-range order to determine structures and is an outstanding approach for specific studies of amorphous materials, which usually display vital line broadening owing to their conformational distribution.22 The Ss 13C NMR spectra of amorphous samples, including amorphous LH, amorphous REP, APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CM (1
1) and CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), are shown in Fig. 7. With the help of ACD/Labs NMR Predictors software (Advanced Chemistry Development, Inc., Toronto, Canada), the positions of carbons in polar groups (which potentially participate in intermolecular interactions) in LH and REP were confirmed and are marked in the Ss 13C NMR spectra (Table S1 in ESI†). In the case of amorphous LH, the single resonance detected at 181.11 ppm corresponded to the signals of carbons 25 and 31 in the carbonyl groups (Fig. 7a and 1). On the other hand, the resonances at 170.59 ppm and 158.11 ppm in the Ss 13C NMR spectrum of REP were assigned to carbon 15 in the secondary amide group and carbon 31 in the carboxyl group, respectively (Fig. 7b). APM (1
1), are shown in Fig. 7. With the help of ACD/Labs NMR Predictors software (Advanced Chemistry Development, Inc., Toronto, Canada), the positions of carbons in polar groups (which potentially participate in intermolecular interactions) in LH and REP were confirmed and are marked in the Ss 13C NMR spectra (Table S1 in ESI†). In the case of amorphous LH, the single resonance detected at 181.11 ppm corresponded to the signals of carbons 25 and 31 in the carbonyl groups (Fig. 7a and 1). On the other hand, the resonances at 170.59 ppm and 158.11 ppm in the Ss 13C NMR spectrum of REP were assigned to carbon 15 in the secondary amide group and carbon 31 in the carboxyl group, respectively (Fig. 7b). APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displayed a spectrum with overlapping carbon signals of amorphous LH and REP (Fig. 7c). In comparison to APM (1
1) displayed a spectrum with overlapping carbon signals of amorphous LH and REP (Fig. 7c). In comparison to APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CM (1
1), CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited very slight resonance shifts in the characteristic carbon signals of LH and REP (i.e., 181.07 → 179.67 ppm (carbonyl groups in LH), 169.97 → 170.26 ppm (secondary amide group in REP) and 158.20 → 158.00 ppm (carboxylic group in REP)) associated with an obviously broadened pattern (Fig. 7d), which might be due to the wide range of molecular orientations and homogeneous dispersion of the two components after coamorphization.22 Such similarities in the Ss 13C NMR spectra of APM (1
1) exhibited very slight resonance shifts in the characteristic carbon signals of LH and REP (i.e., 181.07 → 179.67 ppm (carbonyl groups in LH), 169.97 → 170.26 ppm (secondary amide group in REP) and 158.20 → 158.00 ppm (carboxylic group in REP)) associated with an obviously broadened pattern (Fig. 7d), which might be due to the wide range of molecular orientations and homogeneous dispersion of the two components after coamorphization.22 Such similarities in the Ss 13C NMR spectra of APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CM (1
1) and CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) suggested a similar molecular environment without molecular interaction between the two components. On the basis of the results from FTIR, Raman spectroscopy and Ss 13C NMR, it could be speculated that no specific intermolecular interactions exist in the coamorphous LH–REP systems.
1) suggested a similar molecular environment without molecular interaction between the two components. On the basis of the results from FTIR, Raman spectroscopy and Ss 13C NMR, it could be speculated that no specific intermolecular interactions exist in the coamorphous LH–REP systems.
3.5. pH-solubility profiles
The pH-solubility profiles of crystalline LH, crystalline REP, amorphous LH, amorphous REP, PM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), APM (1
1), APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and CM (1
1), and CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in PBS (0.05 M, pH 2–6.8) are shown in Fig. 8. Crystalline LH and amorphous LH exhibited pH-dependent solubility behavior with maximum solubility at a pH of 3.8, which could be attributed to a pHmax effect.21 Crystalline REP and amorphous REP displayed the lowest solubility at a pH of 5.0 owing to the zwitterionic structure of REP with a carboxylic acid group and a tertiary amine group.51 PM (1
1) in PBS (0.05 M, pH 2–6.8) are shown in Fig. 8. Crystalline LH and amorphous LH exhibited pH-dependent solubility behavior with maximum solubility at a pH of 3.8, which could be attributed to a pHmax effect.21 Crystalline REP and amorphous REP displayed the lowest solubility at a pH of 5.0 owing to the zwitterionic structure of REP with a carboxylic acid group and a tertiary amine group.51 PM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited a solubility profile that almost overlapped with those of the individual crystalline drugs, which indicated no enhancement/decrease in solubility in the presence of the other drug. However, APM (1
1) exhibited a solubility profile that almost overlapped with those of the individual crystalline drugs, which indicated no enhancement/decrease in solubility in the presence of the other drug. However, APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displayed a higher solubility profile than those of amorphous LH and amorphous REP with significantly enhanced solubility in the studied pH range, which indicated that amorphous LH and amorphous REP in APM (1
1) displayed a higher solubility profile than those of amorphous LH and amorphous REP with significantly enhanced solubility in the studied pH range, which indicated that amorphous LH and amorphous REP in APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) might act as recrystallization inhibitors on each other by a steric effect.21,52
1) might act as recrystallization inhibitors on each other by a steric effect.21,52
After coamorphization, both LH and REP in CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) shared similar solubility profiles to those in APM (1
1) shared similar solubility profiles to those in APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which was probably due to the similar physicochemical molecular environments in APMs and CMs, as was characterized and discussed above. A previously reported coamorphous LH-saccharin (1
1), which was probably due to the similar physicochemical molecular environments in APMs and CMs, as was characterized and discussed above. A previously reported coamorphous LH-saccharin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system exhibited a distinct solubility profile with pH-independent properties in the pH range of 2–5.5.21 A coamorphization effect and charge-assisted hydrogen bonding between the N+–H group in LH and the C
1) system exhibited a distinct solubility profile with pH-independent properties in the pH range of 2–5.5.21 A coamorphization effect and charge-assisted hydrogen bonding between the N+–H group in LH and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the coamorphous coformer saccharin in the coamorphous system was speculated to be responsible for such pH-independent solubility behavior. However, in the current study, LH in CM (1
O group in the coamorphous coformer saccharin in the coamorphous system was speculated to be responsible for such pH-independent solubility behavior. However, in the current study, LH in CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) without hydrogen bonding interactions with the coamorphous coformer REP retained the same pH-dependent solubility profile as that of crystalline/amorphous LH. This study demonstrated that charge-assisted hydrogen bonding between LH and a coformer might be pivotal for achieving pH-independent solubility behavior of LH, and a coamorphous system such as LH–REP without intermolecular interactions could not resolve the pH-dependent solubility issue of LH.
1) without hydrogen bonding interactions with the coamorphous coformer REP retained the same pH-dependent solubility profile as that of crystalline/amorphous LH. This study demonstrated that charge-assisted hydrogen bonding between LH and a coformer might be pivotal for achieving pH-independent solubility behavior of LH, and a coamorphous system such as LH–REP without intermolecular interactions could not resolve the pH-dependent solubility issue of LH.
3.6. Intrinsic dissolution profiles
The intrinsic dissolution profiles of LH and REP in CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in comparison with those of amorphous LH, amorphous REP and APM (1
1) in comparison with those of amorphous LH, amorphous REP and APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are shown in Fig. 9. Amorphous LH exhibited a biphasic dissolution profile with two IDRs of 0.0575 (initial 15 min) and 0.0098 (after 15 min) mg cm−2 min−1 owing to recrystallization during dissolution, as was discussed previously.21 After being mixed with amorphous REP, amorphous LH in APM (1
1) are shown in Fig. 9. Amorphous LH exhibited a biphasic dissolution profile with two IDRs of 0.0575 (initial 15 min) and 0.0098 (after 15 min) mg cm−2 min−1 owing to recrystallization during dissolution, as was discussed previously.21 After being mixed with amorphous REP, amorphous LH in APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displayed a delayed biphasic dissolution profile of LH with a turning point at 60 min (Fig. 9A). In contrast to amorphous LH and APM (1
1) displayed a delayed biphasic dissolution profile of LH with a turning point at 60 min (Fig. 9A). In contrast to amorphous LH and APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CM (1
1), CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited a linear release profile of LH with a single IDR of 0.0192 mg cm−2 min−1. At the end of intrinsic dissolution, the tablets were collected and gently touched with tissue paper to remove water on their surface. Powders on the surface of the tablets were gently scraped off and put under a polarized light microscope. Optical phenomena of birefringence were observed in the surface powders from all three types of tablet. It was found that amorphous LH powder almost completely recrystallized, whereas APM (1
1) exhibited a linear release profile of LH with a single IDR of 0.0192 mg cm−2 min−1. At the end of intrinsic dissolution, the tablets were collected and gently touched with tissue paper to remove water on their surface. Powders on the surface of the tablets were gently scraped off and put under a polarized light microscope. Optical phenomena of birefringence were observed in the surface powders from all three types of tablet. It was found that amorphous LH powder almost completely recrystallized, whereas APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CM (1
1) and CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) partially recrystallized with proportions of around 30% and 10%, respectively (see Fig. S1 in ESI†). The lower degree of recrystallization of CM (1
1) partially recrystallized with proportions of around 30% and 10%, respectively (see Fig. S1 in ESI†). The lower degree of recrystallization of CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) might be attributed to its homogeneous intimate mixing into a single phase with a stronger steric effect than that in APM (1
1) might be attributed to its homogeneous intimate mixing into a single phase with a stronger steric effect than that in APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1),13,53 which indicated that coamorphization would benefit the physical stability of amorphous materials via inhibition of recrystallization.
1),13,53 which indicated that coamorphization would benefit the physical stability of amorphous materials via inhibition of recrystallization.
On the other hand, only trace amounts of REP could be determined in the first 40 min, with a cumulative released amount of 0.1033 mg cm−2 at the end of the intrinsic dissolution of crystalline REP. However, amorphous REP underwent much more rapid dissolution with an IDR of 0.0022 mg cm−2 min−1 (Fig. 9B), which could be attributed to its lack of long-range molecular order and higher Gibbs free energy than its crystalline counterpart.54 In comparison to amorphous REP, APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CM (1
1) and CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited 7.5- and 5.6-fold increases in the IDR of REP (Fig. 9B), respectively, which was primarily due to the increased dispersion of REP in the amorphous state.17
1) exhibited 7.5- and 5.6-fold increases in the IDR of REP (Fig. 9B), respectively, which was primarily due to the increased dispersion of REP in the amorphous state.17
Löbmann et al. reported the synchronized release of the two NSAIDs indomethacin and naproxen after coamorphization, and the formation of a heterodimer was speculated to be responsible for such a specific release profile.15 In this study, the release of LH and REP from CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was not synchronized (two separate dissolution profiles, as shown in Fig. S2 in ESI†), which might be due to the absence of intermolecular interactions and the large difference in solubility between these two drugs in the dissolution medium (Fig. 8). However, the relevant factors involved in the synchronized dissolution of two components in a coamorphous system are complicated and worthy of further investigation utilizing more coamorphous systems in future works.
1) was not synchronized (two separate dissolution profiles, as shown in Fig. S2 in ESI†), which might be due to the absence of intermolecular interactions and the large difference in solubility between these two drugs in the dissolution medium (Fig. 8). However, the relevant factors involved in the synchronized dissolution of two components in a coamorphous system are complicated and worthy of further investigation utilizing more coamorphous systems in future works.
3.7. Concentration–time profiles under supersaturated conditions
A study of dissolution under supersaturated conditions was carried out in order to measure the metastable solubilities of both LH and REP in a coamorphous system, and to determine the length of time during which they remained at a supersaturated concentration prior to recrystallization. The supersaturated dissolution profiles of crystalline LH, crystalline REP, amorphous LH, amorphous REP, APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CM (1
1) and CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are shown in Fig. 10. Both crystalline LH and REP exhibited very slow and slight dissolution and reached their equilibrium solubilities within 1 h. Amorphous LH and amorphous REP displayed much higher metastable solubilities during the first hour of supersaturated dissolution, followed by a sharp decline to approach the dissolution profiles of their crystalline counterparts. Such a significant decline in dissolution is a consequence of the precipitation of a more stable but much less soluble crystalline form (the proportion of birefringence in solid samples collected during supersaturated dissolution tests on amorphous LH and amorphous REP gradually increased with time; see Fig. S3 in ESI†), which suggested that supersaturation was the main driving force of nucleation and growth.55 The saturated concentration achieved in the first hour of dissolution led to the reprecipitation of crystalline particles from the solution.56
1) are shown in Fig. 10. Both crystalline LH and REP exhibited very slow and slight dissolution and reached their equilibrium solubilities within 1 h. Amorphous LH and amorphous REP displayed much higher metastable solubilities during the first hour of supersaturated dissolution, followed by a sharp decline to approach the dissolution profiles of their crystalline counterparts. Such a significant decline in dissolution is a consequence of the precipitation of a more stable but much less soluble crystalline form (the proportion of birefringence in solid samples collected during supersaturated dissolution tests on amorphous LH and amorphous REP gradually increased with time; see Fig. S3 in ESI†), which suggested that supersaturation was the main driving force of nucleation and growth.55 The saturated concentration achieved in the first hour of dissolution led to the reprecipitation of crystalline particles from the solution.56
APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gave rise to distinct dissolution profiles of both LH and REP in comparison to those of their amorphous forms alone. After rapidly reaching dissolution peaks of LH and REP at about 2 h, APM (1
1) gave rise to distinct dissolution profiles of both LH and REP in comparison to those of their amorphous forms alone. After rapidly reaching dissolution peaks of LH and REP at about 2 h, APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displayed a relatively smooth decline until the end of dissolution (24 h), still with 5–7 times the equilibrium solubility of LH and REP (Fig. 10). Such an improvement in achieving and maintaining supersaturated levels of LH and REP in the dissolution medium could be attributed to the inhibition of recrystallization by another amorphous component, as was discussed in Section 3.5.
1) displayed a relatively smooth decline until the end of dissolution (24 h), still with 5–7 times the equilibrium solubility of LH and REP (Fig. 10). Such an improvement in achieving and maintaining supersaturated levels of LH and REP in the dissolution medium could be attributed to the inhibition of recrystallization by another amorphous component, as was discussed in Section 3.5.
In contrast to amorphous LH and REP, as well as APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CM (1
1), CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited quite different dissolution behavior, with no significant decrease in supersaturated dissolution over 24 h for either drug. During the first 4 hours, the dissolution of LH and REP from APM (1
1) exhibited quite different dissolution behavior, with no significant decrease in supersaturated dissolution over 24 h for either drug. During the first 4 hours, the dissolution of LH and REP from APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was higher than that from CM (1
1) was higher than that from CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which should be due to the higher IDRs of APM (1
1), which should be due to the higher IDRs of APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), as was observed above (Fig. 9). However, CM (1
1), as was observed above (Fig. 9). However, CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) maintained the supersaturated levels of both drugs after 4 h, whereas APM (1
1) maintained the supersaturated levels of both drugs after 4 h, whereas APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited an obvious decline in the dissolution of both components. This could be ascribed to the fact that the recrystallization rate of APM (1
1) exhibited an obvious decline in the dissolution of both components. This could be ascribed to the fact that the recrystallization rate of APM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was higher than that of CM (1
1) was higher than that of CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) during dissolution, as was discussed above (biphasic intrinsic dissolution profile and Fig. S1 in ESI†).
1) during dissolution, as was discussed above (biphasic intrinsic dissolution profile and Fig. S1 in ESI†).
Supersaturation is critical for enhancing the oral delivery of poorly soluble drugs, because higher supersaturation provides a larger amount of the free soluble drug in the gastrointestinal tract for absorption.20 The average metastable solubilities of LH and REP from 2 h to 24 h achieved for CM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were 5.1 and 8.3 times higher than the equilibrium solubilities of crystalline LH and crystalline REP, respectively. Such an enormous increase in dissolution would facilitate the absorption of both BCS class II drugs and consequently favor their bioavailability.
1) were 5.1 and 8.3 times higher than the equilibrium solubilities of crystalline LH and crystalline REP, respectively. Such an enormous increase in dissolution would facilitate the absorption of both BCS class II drugs and consequently favor their bioavailability.
3.8. Physical stability of solid
From a thermodynamic point of view, the amorphous phase is in a higher free energy state relative to that of the native crystalline phase. There is a thermodynamic driving force of crystallization over time and under stress (temperature, humidity, etc.), and thus the physical stability of amorphous systems usually receives more attention during drug development.6 The XRPD patterns of single amorphous LH and REP, APMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and CMs (2
2) and CMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) before and after storage at 25 °C and 60% RH are presented in Fig. 11.
2) before and after storage at 25 °C and 60% RH are presented in Fig. 11.
Characteristic diffraction peaks of crystalline LH were observed for amorphous LH after storage for 2 days at 25 °C and 60% RH, whereas amorphous REP displayed much greater stability, as no crystalline diffraction peaks appeared even after 90 days in the same conditions. The value of Tg is often used to make judgments about the likely physical stability of amorphous systems.6 In our study, however, amorphous REP, which had a lower Tg (53.35 °C), displayed greater physical stability than amorphous LH (Tg = 67.43 °C). Basically, crystallization, as the result of two independent phenomena, namely, nucleation and crystal growth, is characterized by a lag time before the formation of stable nuclei followed by the growth of crystals from these stable nuclei.7 Factors that affect nucleation and/or crystal growth would have an effect on the overall behavior of crystallization. Parameters related to crystallization from the amorphous state can be categorized as: (1) thermodynamic properties (i.e., configuration entropy, enthalpy or Gibbs free energy); (2) kinetic properties (i.e., molecular mobility, indicated by the Tg value or the structural relaxation time); and (3) the molecular environment (e.g., hydrogen bonding interactions, π–π stacking, etc.).7,57 In addition, independently of the drug molecule itself, environmental stress (e.g., temperature, humidity, and mechanical stress), preparation methods and conditions have also been reported to influence crystallization behavior.58–60 It is challenging to directly predict the relationship between the Tg value and the stability of amorphous materials. For instance, Fukuoka et al. observed that amorphous indomethacin was more stable than amorphous phenobarbital even though both compounds had the same Tg.61 A similar trend was observed by Marsac et al. for amorphous felodipine, which had much greater stability than amorphous nifedipine.62 The greater ease of the crystallization of nifedipine from its amorphous form was attributed to its higher thermodynamic driving force (configuration enthalpy) of nucleation, which was proved by observing the nucleation rate of amorphous drugs at a temperature below their Tg while excluding the influence of humidity (0% RH). In the case of amorphous LH and REP, characteristic peaks of crystalline LH were observed by XRPD after storage for 2 days in an environment at 25 °C and 0% RH, whereas REP remained in its amorphous state (Fig. S4 in ESI†), which indicated the faster nucleation rate and greater ease of recrystallization of LH, which was possibly due to its intrinsic strong thermodynamic driving force, as was shown in the case of nifedipine. In addition, the moisture content would also affect the crystallization behavior of amorphous drugs. It has been well established that water absorbed by an amorphous material would act as a plasticizer to lower the Tg value of the solid and increase its molecular mobility, thus accelerating the rate and extent of the transformation process. With an increase in the water content, the experimental Tg values of both polymers and low-molecular-weight drugs or excipients decreased and well fitted the Gordon–Taylor equation (eqn (1)).63 In the present study, the moisture contents of amorphous LH and amorphous REP after storage for one day at 25 °C and 80% RH (saturated ammonium sulfate solution in a sealed container) were determined to be 2.27% and 0.45% by the Karl Fischer method (Volumetric KF Titrator V20, Mettler Toledo, Switzerland), respectively. In addition, the Tg values of amorphous LH and amorphous REP after the absorption of moisture were determined to be 37.1 °C and 49.4 °C, respectively. The higher hygroscopicity of amorphous LH lowered its Tg to a much greater extent than that of amorphous REP, which resulted in the lower physical stability of amorphous LH.
For APMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Fig. 11c–e), nine diffraction peaks at 8.9°, 11.6°, 13.8°, 15.1°, 16.4°, 17.1°, 19.5°, 20.7° and 21.9° (2θ) were detected after storage for 2 days at 25 °C and 60% RH, which should belong to crystalline LH (without observation of the characteristic diffraction peaks of crystalline REP at 7.59°, 10.06°, 20.23° and 30.81°). Although APMs exhibited higher IDRs and supersaturated dissolutions of both LH and REP in the initial period (Fig. 9 and 10), they could not prevent devitrification of the amorphous phase over its shelf life. In contrast, no characteristic diffraction peaks of crystalline LH or REP were detected for CMs (2
2) (Fig. 11c–e), nine diffraction peaks at 8.9°, 11.6°, 13.8°, 15.1°, 16.4°, 17.1°, 19.5°, 20.7° and 21.9° (2θ) were detected after storage for 2 days at 25 °C and 60% RH, which should belong to crystalline LH (without observation of the characteristic diffraction peaks of crystalline REP at 7.59°, 10.06°, 20.23° and 30.81°). Although APMs exhibited higher IDRs and supersaturated dissolutions of both LH and REP in the initial period (Fig. 9 and 10), they could not prevent devitrification of the amorphous phase over its shelf life. In contrast, no characteristic diffraction peaks of crystalline LH or REP were detected for CMs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Fig. 11f–h). Considering the detection sensitivity of XRPD, polarized light microscopy was used to observe the possible formation of small amounts of crystals in samples stored for 90 days. No birefringence was observed (Fig. S5 in ESI†), which indicates the absence of a phase transition of coamorphous LH–REP systems and their high physical stability. In comparison to amorphous LH and APMs, the enhanced physical stability of CMs (single-phase amorphous binary systems) could be attributed to intimate mixing at the molecular level with homogeneous steric effects, which theoretically decreases the difference in free energy between crystalline and amorphous materials and inhibits crystallization.13
2) (Fig. 11f–h). Considering the detection sensitivity of XRPD, polarized light microscopy was used to observe the possible formation of small amounts of crystals in samples stored for 90 days. No birefringence was observed (Fig. S5 in ESI†), which indicates the absence of a phase transition of coamorphous LH–REP systems and their high physical stability. In comparison to amorphous LH and APMs, the enhanced physical stability of CMs (single-phase amorphous binary systems) could be attributed to intimate mixing at the molecular level with homogeneous steric effects, which theoretically decreases the difference in free energy between crystalline and amorphous materials and inhibits crystallization.13
3.9. Chemical stability
In comparison to the chromatograms of the original crystalline LH and REP (Fig. S6A and B†), no extra degradation peak appeared in the chromatogram of freshly prepared coamorphous LH–REP, as well as that of the coamorphous LH–REP sample, after storage for 90 days under conditions of 25 °C and 60% RH (Fig. S6E†). In comparison with the impurities present in the original crystalline LH (peak 1) and crystalline REP (peak 1′), there was no significant increase after the coamorphization of LH and REP, as well as in coamorphous LH–REP, after storage at 25 °C and 60% RH for 90 days (Table S2†). However, the total amount of impurities in coamorphous LH–REP significantly increased upon storage at temperatures of 40 °C and 60 °C for 10 days, which was mainly due to an increase in REP impurities (peak 1′ and peak 2′) (Table S2†). Moreover, no significant difference in the total amount of impurities was detected between coamorphous LH–REP and APM, which indicated that the coamorphization process would not affect the degradation of REP at higher temperatures (i.e., 40 °C and 60 °C).In addition, after storage for 90 days at 25 °C and 60% RH, the contents of LH and REP in CMs were determined to be close to 100% (99.02–100.23%, Fig. S7 in ESI†), which indicated the high stability of the coamorphous system.64
Currently, most reports on coamorphous systems have only compared their physicochemical characteristics, solubility/dissolution properties and/or physical stabilities with those of their single crystalline/amorphous components.15,17–19,43,65,66 In the present study, in addition to comparisons with the single crystalline/amorphous components, comprehensive comparisons in various respects between the coamorphous systems and amorphous physical mixtures of both components were also undertaken, which facilitated the clarification of the differences between these two systems (single-phase binary system vs. two-phase binary system) in solubility/dissolution and physical stability.
4. Conclusion
This study was a follow-up of research on previously reported coamorphous LH–saccharin with a charge-assisted hydrogen bond between the N+–H group of LH and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of saccharin. In this study, coamorphous LH–REP systems were intentionally created and confirmed to be a comparable system without intermolecular interactions, and the N+–H group of LH was intact. They exhibited significantly enhanced intrinsic and supersaturation dissolution of both components, as well as physical stability, while retaining the pH-dependent solubility profile of LH. Intermolecular interaction with the N+–H group in the LH structure was concluded to be essential to improve the pH-dependent solubility behavior of LH. This demonstrated that molecular interactions may be intentionally designed by using different hydrogen donors or receptors as coamorphous coformers, to enable/favor the enhancement of specific physicochemical properties.
O group of saccharin. In this study, coamorphous LH–REP systems were intentionally created and confirmed to be a comparable system without intermolecular interactions, and the N+–H group of LH was intact. They exhibited significantly enhanced intrinsic and supersaturation dissolution of both components, as well as physical stability, while retaining the pH-dependent solubility profile of LH. Intermolecular interaction with the N+–H group in the LH structure was concluded to be essential to improve the pH-dependent solubility behavior of LH. This demonstrated that molecular interactions may be intentionally designed by using different hydrogen donors or receptors as coamorphous coformers, to enable/favor the enhancement of specific physicochemical properties.
5. Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This research was supported by the National Natural Science Foundation of China (NO. 81202988), the Natural Science Foundation of Jiangsu Province (BK20130659, BK20141351, BK20151438), the Fundamental Research Funds for the Central Universities (ZJ16088), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Qing Lan Project of Jiangsu Province.References
- L. Di, P. V. Fish and T. Mano, Drug Discovery Today, 2012, 17, 486–495 CrossRef CAS PubMed.
- D. Engers, J. Teng, J. Jimenez-Novoa, P. Gent, S. Hossack, C. Campbell, J. Thomson, I. Ivanisevic, A. Templeton, S. Byrn and A. Newman, J. Pharm. Sci., 2010, 99, 3901–3922 CrossRef CAS PubMed.
- A. M. Kaushal, P. Gupta and A. K. Bansal, Crit. Rev. Ther. Drug Carrier Syst., 2004, 21, 133–193 CrossRef CAS PubMed.
- D. E. Alonzo, G. G. Z. Zhang, D. Zhou, Y. Gao and L. S. Taylor, Pharm. Res., 2010, 27, 608–618 CrossRef CAS PubMed.
- K. Grzybowska, M. Paluch, A. Grzybowski, Z. Wojnarowska, L. Hawelek, K. Kolodziejczyk and K. L. Ngai, J. Phys. Chem. B, 2010, 114, 12792–12801 CrossRef CAS PubMed.
- J. A. Baird and L. S. Taylor, Adv. Drug Delivery Rev., 2012, 64, 396–421 CrossRef CAS PubMed.
- R. Laitinen, K. Lobmann, C. J. Strachan, H. Grohganz and T. Rades, Int. J. Pharm., 2013, 453, 65–79 CrossRef CAS PubMed.
- P. Gupta, V. K. Kakumanu and A. K. Bansal, Pharm. Res., 2004, 21, 1762–1769 CrossRef CAS.
- F. Wang, H. Hui, T. J. Barnes, C. Barnett and C. A. Prestidge, Mol. Pharm., 2010, 7, 227–236 CrossRef CAS PubMed.
- A. T. M. Serajuddin, J. Pharm. Sci., 1999, 88, 1058–1066 CrossRef CAS PubMed.
- C. A. Prestidge, T. J. Barnes, C. H. Lau, C. Barnett, A. Loni and L. Canham, Expert Opin. Drug Delivery, 2007, 4, 101–110 CrossRef CAS PubMed.
- T. Vasconcelos, B. Sarmento and P. Costa, Drug Discovery Today, 2007, 12, 1068–1075 CrossRef CAS PubMed.
- S. J. Dengale, H. Grohganz, T. Rades and K. Lobmann, Adv. Drug Delivery Rev., 2016, 100, 116–125 CrossRef CAS PubMed.
- H. H. Guo, N. N. Miao, T. F. Li, J. Hao, Y. Gao and J. J. Zhang, Progr. Chem., 2014, 26, 478–486 CAS.
- K. Löbmann, R. Laitinen, H. Grohganz, K. C. Gordon, C. Strachan and T. Rades, Mol. Pharm., 2011, 8, 1919–1928 CrossRef PubMed.
- K. Löbmann, C. Strachan, H. Grohganz, T. Rades, O. Korhonen and R. Laitinen, Eur. J. Pharm. Biopharm., 2012, 81, 159–169 CrossRef PubMed.
- N. Chieng, J. Aaltonen, D. Saville and T. Rades, Eur. J. Pharm. Biopharm., 2009, 71, 47–54 CrossRef CAS PubMed.
- S. Yamamura, H. Gotoh, Y. Sakamoto and Y. Momose, Int. J. Pharm., 2002, 241, 213–221 CrossRef CAS PubMed.
- A. Teja, P. B. Musmade, A. B. Khade and S. J. Dengale, Eur. J. Pharm. Sci., 2015, 78, 234–244 CrossRef CAS PubMed.
- Y. Gao, J. Liao, X. Qi and J. Zhang, Int. J. Pharm., 2013, 450, 290–295 CrossRef CAS PubMed.
- S. Qian, W. Heng, Y. Wei, J. Zhang and Y. Gao, Cryst. Growth Des., 2015, 15, 2920–2928 CAS.
- S. Schantz, P. Hoppu and A. M. Juppo, J. Pharm. Sci., 2009, 98, 1862–1870 CrossRef CAS PubMed.
- T. Masuda, Y. Yoshihashi, E. Yonemochi, K. Fujii, H. Uekusa and K. Terada, Int. J. Pharm., 2012, 422, 160–169 CrossRef CAS PubMed.
- K. Löbmann, H. Grohganz, R. Laitinen, C. Strachan and T. Rades, Eur. J. Pharm. Biopharm., 2013, 85, 873–881 CrossRef PubMed.
- K. Löbmann, R. Laitinen, C. Strachan, T. Rades and H. Grohganz, Eur. J. Pharm. Biopharm., 2013, 85, 882–888 CrossRef PubMed.
- H. A. Nasrallah, R. Silva, D. Phillips, J. Cucchiaro, J. Hsu, J. Xu and A. Loebel, J. Psychiatr. Res., 2013, 47, 670–677 CrossRef PubMed.
- M. Ankit, Y. Manish, C. Dinesh and S. Birendra, Int. J. Res. Ayurveda Pharm., 2014, 5, 632–637 CrossRef CAS.
- E. Gagnière, D. Mangin, F. Puel, A. Rivoire, O. Monnier, E. Garcia and J. P. Klein, J. Cryst. Growth, 2009, 311, 2689–2695 CrossRef.
- D. Cohen, R. P. Stolk, D. E. Grobbee and C. C. Gispen-de Wied, Diabetes Care, 2006, 29, 786–791 CrossRef PubMed.
- J. Knapik, Z. Wojnarowska, K. Grzybowska, K. Jurkiewicz, L. Tajber and M. Paluch, Mol. Pharm., 2015, 12, 3610–3619 CrossRef CAS PubMed.
- K. Rajagopalan, S. Guo, L. Hernandez, J. Green and A. Loebel, Open Med. J., 2014, 1, 1–9 CrossRef.
- D. W. Haupt and J. W. Newcomer, J. Clin. Psychiatry, 2001, 62, 15–26 CAS.
- A. Annamalai and C. Tek, Int. J. Endocrinol., 2015, 2015, 969182 Search PubMed.
- M. J. McNeely, Clin. Diabetes, 2002, 20, 195–196 CrossRef.
- M. E. J. Lean and F.-G. Pajonk, Diabetes Care, 2003, 26, 1597–1605 CrossRef CAS PubMed.
- C. R. Culy and B. Jarvis, Drugs, 2001, 61, 1625–1660 CrossRef CAS PubMed.
- A. Forster, J. Hempenstall and T. Rades, Drug Dev. Ind. Pharm., 2001, 27, 549–560 CrossRef CAS PubMed.
- F. Qian, J. Huang and M. A. Hussain, J. Pharm. Sci., 2010, 99, 2941–2947 CrossRef CAS PubMed.
- J. Zhang, D. Liu, Y. Huang, Y. Gao and S. Qian, Int. J. Pharm., 2012, 436, 311–317 CrossRef CAS PubMed.
- R. Chadha, S. Bhandari, P. Arora and R. Chhikara, Pharm. Dev. Technol., 2013, 18, 504–514 CrossRef CAS PubMed.
- C. Hock, S. Straburg, H. Haberland and M. Schemidt, Phys. Rev. Lett., 2008, 101, 023401 CrossRef CAS PubMed.
- A. Forster, J. Hempenstall and T. Rades, J. Pharm. Pharmacol., 2001, 53, 303–315 CrossRef CAS PubMed.
- S. J. Dengale, O. P. Ranjan, S. S. Hussen, B. S. M. Krishna, P. B. Musmade, G. G. Shenoy and K. Bhat, Eur. J. Pharm. Sci., 2014, 62, 57–64 CrossRef CAS PubMed.
- M. Allesø, N. Chieng, S. Rehder, J. Rantanen, T. Rades and J. Aaltonen, J. Controlled Release, 2009, 136, 45–53 CrossRef PubMed.
- L. S. Taylor and G. Zografi, J. Pharm. Sci., 1998, 87, 1615–1621 CrossRef CAS PubMed.
- R. Nair, N. Nyamweya, S. Gönen, L. J. Martínez-Miranda and S. W. Hoag, Int. J. Pharm., 2001, 225, 83–96 CrossRef CAS PubMed.
- A. Heinz, C. J. Strachan, K. C. Gordon and T. J. Rades, J. Pharm. Pharmacol., 2009, 61, 971–988 CrossRef CAS PubMed.
- A. Beyer, L. Radi, H. Grohganz, K. Lobmann, T. Rades and C. S. Leopold, Eur. J. Pharm. Biopharm., 2016, 104, 72–81 CrossRef CAS PubMed.
- L. Xu, C. Li and K. Y. S. Ng, J. Phys. Chem. A, 2000, 104, 3952–3957 CrossRef CAS.
- R. T. Berendt, D. M. Sperger, E. J. Munson and P. K. Isbester, Trends Anal. Chem., 2006, 25, 977–984 CrossRef CAS.
- Z. Mandić and V. Gabelica, J. Pharm. Biomed. Anal., 2006, 41, 866–871 CrossRef PubMed.
- J. H. Kim and H. K. Choi, Int. J. Pharm., 2002, 236, 81–85 CrossRef CAS PubMed.
- O. Korhonen, K. Pajula and R. Laitinen, Expert Opin. Drug Delivery, 2016, 1–19, DOI:10.1080/17425247.2016.1198770.
- A. A. Elamin, C. Ahlneck, G. Alderborn and C. Nystrom, Int. J. Pharm., 1994, 111, 159–170 CrossRef CAS.
- T. Purvis, M. E. Mattucci, M. T. Crisp, K. P. Johnston and R. O. Williams, AAPS PharmSciTech, 2007, 8, E58 CrossRef PubMed.
- S. Maruyama and H. Ooshima, J. Cryst. Growth, 2000, 212, 239–245 CrossRef CAS.
- C. Bhugra and M. J. Pikal, J. Pharm. Sci., 2008, 97, 1329–1349 CrossRef CAS PubMed.
- C. Bhugra, R. Shmeis and M. J. Pikal, J. Pharm. Sci., 2008, 97, 4446–4458 CrossRef CAS PubMed.
- P. Karmwar, K. Graeser, K. C. Gordon, C. J. Strachan and T. Rades, Int. J. Pharm., 2011, 417, 94–100 CrossRef CAS PubMed.
- M. Ohta and G. Buckton, Int. J. Pharm., 2005, 289, 31–38 CrossRef CAS PubMed.
- E. Fukuoka, M. Makita and Y. Nakamura, Chem. Pharm. Bull., 1989, 37, 2782–2785 CrossRef CAS PubMed.
- P. J. Marsac, H. Konno and L. S. Taylor, Pharm. Res., 2006, 23, 2306–2316 CrossRef CAS PubMed.
- B. C. Hancock and G. Zografi, Pharm. Res., 1994, 11, 471–477 CrossRef CAS.
- U. S. Food and Drug Administration, Guidance for Industry: Regulatory Classification of Pharmaceutical Co-crystals, April 2013 Search PubMed.
- S. J. Dengale, S. S. Hussen, B. S. Krishna, P. B. Musmade, G. Gautham Shenoy and K. Bhat, Eur. J. Pharm. Biopharm., 2015, 89, 329–338 CrossRef CAS PubMed.
- S. Wairkar and R. Gaud, AAPS PharmSciTech, 2016, 17, 673–681 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: More photomicrographs by polarizing microscope and XRPD are provided in the ESI. See DOI: 10.1039/c6ra18022a |
| ‡ S. Qian and Z. Li contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |









