Preparation, properties, and efficient electrically induced structure formation of a leaky dielectric photoresist
Guowei Lv a,
Shihu Zhanga,
Jinyou Shaob,
Hongmiao Tianb,
Guolong Wanga and
Demei Yu*a
a,
Shihu Zhanga,
Jinyou Shaob,
Hongmiao Tianb,
Guolong Wanga and
Demei Yu*a
aDepartment of Chemistry, School of Science, State Key Laboratory of Electrical Insulation and Power Equipments, Xi'an Jiaotong University, Xi'an 710049, Shaanxi, P. R. China. E-mail: dmyu@mail.xjtu.edu.cn; Tel: +86 29 82668019
bState Key Laboratory of Manufacturing Systems Engineering, Xi'an Jiaotong University, Xi'an 710049, Shaanxi, P. R. China
First published on 18th August 2016
Abstract
A leaky dielectric photoresist was designed and prepared by doping a soluble conductive polypyrrole into a low-viscosity photocurable resin (perfect dielectric) to achieve efficient electrically induced structure formation (EISF). The comprehensive properties of both the leaky dielectric photoresist and its cured film were systematically investigated. It was found that the leaky dielectric photoresist is homogeneous and stable in both the liquid state and solid state after curing, as a result of the low molecular weight and the bulky side groups of polypyrrole. The leaky dielectric photoresist retains the Newtonian nature of the photocurable resin with low viscosity, displays a significant increase in the electrical conductivity with an increase in the polypyrrole loading, and shows favorable wettability on a silicon substrate. Meanwhile, the cured film is still transparent, thermally stable and featureless, following the increase in the polypyrrole loading. It is worth noting that by using a low-viscosity leaky dielectric photoresist, pillar arrays can be rapidly fabricated over large areas at ambient temperature via EISF onto a featureless template. The resulting patterned film is hydrophobic with an apparent contact angle of 109°, even though the cured film is hydrophilic with an intrinsic contact angle of 64°.
1. Introduction
Electrically induced structure formation (EISF) has been explored as a straightforward, cost-effective and contactless lithography technique for fabricating micro-/nanostructures, where a template and a substrate act as an electrode pair, sandwiching a polymeric film and an air gap.1–13 With a voltage applied to the electrode pair, the destabilizing electrostatic force generated by the electric field at the polymeric film–air interface overcomes the stabilizing surface tension and the viscous resistance. This therefore arouses the flow and deformation of the polymeric film upwards to the template, so as to form a hexagonal pillar array on the substrate.14 The competition between the electrostatic force and surface tension leads to a characteristic wavelength λ, which corresponds to the spacing between pillars.15 In particular, EISF onto a featureless template, unlike some lithography techniques (e.g. UV imprint lithography and hot embossing), avoids complications, time, cost and the shortcomings associated with the diffraction limit in making a pre-patterned template. This technique thereby provides a simple and economical way to fabricate microstructures over large areas for fundamental research as well as for practical applications such as superhydrophobic surfaces, micro-/nanoelectromechanical systems (MEMS/NEMS), chemical sensors, electronic systems, micro-/nanofluidic systems, and so on.16–22Commonly used thermoplastic polymers (with an electrical conductivity smaller than 10−8 S cm−1 that can be considered as perfect dielectrics, such as PS, PMMA, PVDF and PBrS) suffer slow patterning dynamics owing to their high melt viscosity and the need for heat to modulate their rheological properties.23–26 Therefore, endeavors are still needed to fulfill efficient EISF (namely fast replication dynamics, small feature size, near room temperature processing and energy-saving processes). Goldberg-Oppenheimer et al. experimentally demonstrated that the use of low-viscosity thermoplastic polymers significantly reduced the completion time,27 while Dickey et al. showed that photocurable systems with low viscosity formed pillar arrays nearly instantaneously under ambient conditions.14 However, low-viscosity polymers still suffer limitations in the reduction of the length of the structure. Russel et al. theoretically demonstrated that a small characteristic wavelength λ and a short characteristic time τ could be obtained by decreasing the interfacial tension, increasing the dielectric constant or increasing the electrical conductivity of the polymeric fluid, paving the way for smaller pattern sizes, greater aspect ratios, and faster growing dynamics.28–33 For instance, Lin et al. demonstrated that the reduction of interfacial tension, by substituting the air gap with another layer of fluid or polymer, led to a significant reduction in the characteristic wavelength, λ.34–36 Similarly, Bae et al. demonstrated that the incorporation of nanoparticles into the polymeric film increased the dielectric constant of the polymeric film, leading to a systematic reduction in the length scale.37 Furthermore, Goldberg-Oppenheimer et al. experimentally investigated the evolution of a conductive polymeric film under an externally applied voltage.38,39 However, the time scale of these polymeric films is still slow due to their relatively high melt viscosity.
Taking into account the aforementioned research, a dielectric material with fast photocurable behavior, low viscosity, and leaky dielectric properties (with an electrical conductivity larger than 10−6 S cm−1) may be a promising material for efficient EISF. However, common photocurable resins are perfect dielectric materials. Available routes for transforming a perfect dielectric into a leaky dielectric, such as doping conductive fillers into an insulating matrix, have been developed extensively.40–42 However, suitable photocurable leaky dielectric materials for efficient EISF are rare, because loading excessive nanoparticles can result in undesirable nanoparticle agglomeration in a polymeric film, especially during its flow and deformation under an applied external field. This can induce a dramatic increase in the viscosity of the polymeric film, limit further decreases in the scale size of the micro-/nanostructure, and cause a severe reduction in the thermal stability, UV-vis transmittance and surface smoothness of the cured film.43–45
In this paper, a leaky dielectric photoresist was designed and prepared by doping a soluble conductive polypyrrole into a photocurable resin (bisphenol A (4) ethoxylated dimethacrylate, BPA4EODMA) to fulfill efficient EISF. The comprehensive properties of both the leaky dielectric photoresist (homogeneity, stability, rheological behavior, electrical properties and curing process) and its cured film (the thermal stability, UV-vis transmittance and surface roughness) were systematically investigated. Moreover, the leaky dielectric photoresist was selected to preliminarily carry out EISF. In addition, the hydrophobicity of the patterned film and the flat film was investigated.
2. Experimental section
2.1 Materials
BPA4EODMA was purchased from Guangzhou Deco Composite Technology. The soluble conductive polymer of 2-methyl-1,3-di(1H-pyrrol-1-yl)propan-1-one was prepared according to the procedure described elsewhere.46 2-Hydroxy-2-methyl-1-phenyl-1-propanone (1173) was purchased from Zibo Pioneer Import & Export Co. Ltd. Trimethoxy(1H,1H,2H,2H-heptadecafluorodecyl)silane (FAS) was purchased from SICONG chemical Reagent Co. Ltd. Indium tin oxide (ITO)-coated glass slides with a resistance of 8 Ω cm were used as transparent electrodes for UV light transmittance studies. Polyimide (PI) films with a thickness of 25 μm were used as an electrode spacer.2.2 Preparation of the leaky dielectric photoresist
A finite amount of polypyrrole was added into the solvent tetrahydrofuran (0.55 g) and was mechanically stirred for 1 h at room temperature to obtain a series of well-dissolved polypyrrole solutions. Subsequently, BPA4EODMA (0.45 g) and 1173 (75 mg) were added to the prepared polypyrrole solutions, which were then mechanically stirred for 12 h to obtain a series of homogeneous leaky dielectric photoresists. The values of ω (the weight fraction of polypyrrole in the photoresist) obtained for the leaky dielectric photoresists are in the range from 0 to 1.6 wt%. Thin leaky dielectric photoresist films were prepared by spin-coating the leaky dielectric photoresist onto ITO-glass and a Si substrate using a SIYOUYEN KW-4A spin-coater. The thickness of the film was controlled by adjusting the speed and time of the spin-coating. The film was cured using UV light (MEJIRO PRECISION SHG-200) with a wavelength of 253.7 nm.2.3 Characterization
3. Results and discussion
3.1 Preparation of the leaky dielectric photoresist
The uniform distribution of polypyrrole in BPA4EODMA is crucial for fabricating an EISF structure, as this will determine the comprehensive properties of the leaky dielectric photoresist. Specifically, a small-molecule photocurable resin, BPA4EODMA, was first selected as an EISF matrix due to its low viscosity and fast polymerization kinetics. Inspired by previous works,46,47 a soluble conductive polypyrrole derivative was synthesized to serve as a conductive component. The bulky side chains and low molecular weight of this pyrrole polymer result in poor macromolecular regularity and weak intermolecular interactions of the polypyrrole chains, so that the solvent and matrix molecules can readily penetrate into the polypyrrole chains. Moreover, polypyrrole is compatible with BPA4EODMA due to the similar structure both on the polypyrrole side chains and the BPA4EODMA main chains, resulting in strong intermolecular interactions between the polypyrrole chains and matrix molecules, as shown in Fig. 1. Therefore, such a leaky dielectric photoresist is homogeneous and stable in the liquid state. The morphology of the surface and the fracture surface of the cured film with a ω value of 1.2 wt% are shown in Fig. 2. The cured film appears to be homogeneous and featureless, thus confirming the excellent compatibility between polypyrrole and the BPA4EODMA matrix and the favorable thin film-forming properties of the leaky dielectric photoresist on the ITO-substrate. | ||
| Fig. 2 SEM images of (a) the surface and (b) the fracture surface of the cured film with a ω value of 1.2 wt%. | ||
3.2 Properties of the leaky dielectric photoresist and its cured film
To achieve efficient EISF, the properties of the leaky dielectric photoresist are crucial. The rheological properties of the polymeric fluid clearly reveal the variation in the molecular interactions during the flow and deformation under an applied external field,48,49 and remarkably affect the process of EISF as well. Generally, the relatively low viscosity of the polymeric fluid facilitates rapid patterning. The variation of the apparent viscosity and the shear stress vs. the shear rate for the leaky dielectric photoresists with different ω values is shown in Fig. 3. Although ω in the leaky dielectric photoresists reaches as high as 1.6 wt%, all the leaky dielectric photoresists exhibit Newtonian behavior and the apparent viscosity increases slightly in the range of ∼4 to ∼7 mPa s with an increase in the polypyrrole loading. This is attributed to the fact that the polypyrrole chains exist as individual chains,50 and thus the intramolecular interactions between the polypyrrole chains are dominant while the intermolecular interactions are almost absent.51–53 Moreover, rheological studies also indicate that the leaky dielectric photoresist remains relatively stable and homogeneous during flow and deformation. | ||
| Fig. 3 The experimental curves of apparent viscosity (filled symbols), shear stress (hollow symbols) vs. shear rate for the leaky dielectric photoresists with different values of ω. | ||
The electrical conductivity of the leaky dielectric photoresist is a critical factor since it governs the destabilizing electrostatic force at the polymeric fluid–air interface and thus affects the process of structure formation in EISF. Fig. 4 shows the electrical conductivity of the leaky dielectric photoresists with different values of ω. The electrical conductivity of the leaky dielectric photoresist obviously increases with an increase in the polypyrrole loading. The leaky dielectric photoresist without polypyrrole exhibits an electrical conductivity of 0.032 μS cm−1, while the leaky dielectric photoresist with a ω value of 1.6 wt% exhibits an electrical conductivity of 6.36 μS cm−1. This indicates that the perfect dielectric (ω = 0 wt%) is transformed into a leaky dielectric through the doping of polypyrrole into BPA4EODMA. It is worth noting that a low loading of polypyrrole (ω around 0.2 wt%) gives rise to a significant increase in the electrical conductivity from 10−2 μS cm−1 to 1 μS cm−1 for the leaky dielectric photoresist. This is ascribed to the fact that the adequate dispersion of polypyrrole in the leaky dielectric photoresist results in a low percolation threshold for charge transport.54,55 With the formation of an electrical conduction path via electron hopping in the leaky dielectric photoresist, there would be no significant increase in electrical conductivity with a further increase in the polypyrrole loading.
The wettability of the leaky dielectric photoresists with different ω values on the silicon substrate was examined and quantified by the contact angle, as shown in Fig. 5. It was found that the contact angles of the leaky dielectric photoresist on the silicon substrate does not change a lot with an increase in the polypyrrole loading. The leaky dielectric photoresist without polypyrrole shows a maximum contact angle of about 38°, and the leaky dielectric photoresist with a ω value of 1.2 wt% has a minimum contact angle of about 29°. This indicates that the leaky dielectric photoresist has favorable wettability on a silicon substrate.
 | ||
| Fig. 5 The contact angle of the leaky dielectric photoresists with different ω values on a silicon substrate. | ||
The conversion of methacrylate groups in BPA4EODMA with irradiation time was monitored using Fourier transform infrared spectroscopy. The variation in the absorption peak intensity at 1638 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds) for the leaky dielectric photoresist with a ω value of 1.2 wt% at different radiation times is shown in Fig. 6a. It was found that the relative intensity of the absorption peak of the C
C bonds) for the leaky dielectric photoresist with a ω value of 1.2 wt% at different radiation times is shown in Fig. 6a. It was found that the relative intensity of the absorption peak of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds weakens with the extension of radiation time in the range from 0 to 50 s, which indicates the polymerization of BPA4EODMA. In order to obtain a more intuitive reflection of the polymerization, the absorption peak of C
C bonds weakens with the extension of radiation time in the range from 0 to 50 s, which indicates the polymerization of BPA4EODMA. In order to obtain a more intuitive reflection of the polymerization, the absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1719 cm−1 was adopted as an internal reference, and the area ratio of the C
O at 1719 cm−1 was adopted as an internal reference, and the area ratio of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds to the C
O bonds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (AC
C bonds (AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/AC
O/AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) versus the radiation time is shown in Fig. 6b. It was found that the value of AC
C) versus the radiation time is shown in Fig. 6b. It was found that the value of AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/AC
O/AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C shows a slight increase at the first 20 s, followed by a sharp increase within the range of 20–40 s. When the radiation time is extended to 50 s, no further obvious increase in AC
C shows a slight increase at the first 20 s, followed by a sharp increase within the range of 20–40 s. When the radiation time is extended to 50 s, no further obvious increase in AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/AC
O/AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C is observed. The high AC
C is observed. The high AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/AC
O/AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C values correspond to the high proportion of polymerized C
C values correspond to the high proportion of polymerized C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. Fig. 6c shows the reaction rate and reaction extent for the leaky dielectric photoresist film (ω of 1.2 wt%). The reaction rate shows a slight increase within the first 20 s, followed by a sharp increase within the range of 20–30 s (up to 2.47% per s). With further extension of the radiation time, the reaction rate shows a significant reduction. Therefore, it can be concluded that the polymerization of the leaky dielectric photoresist mainly starts after 20 s under UV radiation resulting from oxygen inhibition and is basically completed within 40 s. It is worth noting that the content of polypyrrole in the leaky dielectric photoresist has an obvious effect on the curing rate of the photoresist. As shown in Fig. 6d, both the photoinitiator 1173 and polypyrrole exhibit strong absorption in the range of 280–380 nm. The UV-vis absorption of polypyrrole reduces the light intensity, and therefore slows the polymerization rate of the leaky dielectric photoresist. Therefore, if more polypyrrole is added, a longer curing time is required. The radiation time of 50 s was applied uniformly in our experiments to cure the films with different ω values.
C bonds. Fig. 6c shows the reaction rate and reaction extent for the leaky dielectric photoresist film (ω of 1.2 wt%). The reaction rate shows a slight increase within the first 20 s, followed by a sharp increase within the range of 20–30 s (up to 2.47% per s). With further extension of the radiation time, the reaction rate shows a significant reduction. Therefore, it can be concluded that the polymerization of the leaky dielectric photoresist mainly starts after 20 s under UV radiation resulting from oxygen inhibition and is basically completed within 40 s. It is worth noting that the content of polypyrrole in the leaky dielectric photoresist has an obvious effect on the curing rate of the photoresist. As shown in Fig. 6d, both the photoinitiator 1173 and polypyrrole exhibit strong absorption in the range of 280–380 nm. The UV-vis absorption of polypyrrole reduces the light intensity, and therefore slows the polymerization rate of the leaky dielectric photoresist. Therefore, if more polypyrrole is added, a longer curing time is required. The radiation time of 50 s was applied uniformly in our experiments to cure the films with different ω values.
The thermal stability of the cured films with different values of ω was investigated as shown in Fig. 7a. All films show similar thermogravimetric curves. The small weight loss at around 100 °C can be attributed to the loss of small molecules or moisture in the sample, while the obvious decomposition of the cured films starts at about 370 °C and finishes at around 480 °C. Obviously, the addition of polypyrrole had no significant effect on the thermal stability of the cured films with different ω values. The transmittance spectra of the cured films with different ω values in the UV-vis wavelength range are shown in Fig. 7b. The transmittance of the cured films gradually decreases with an increase in the polypyrrole content owing to the absorption of polypyrrole in the cured films. Additionally, an obvious decline in the transmittance of the cured films is observed in the range of 300–500 nm with an increase in the polypyrrole loading, but the reduction in transmittance is not significant in the visible region.
 | ||
| Fig. 7 (a) Thermogravimetric curves and (b) UV-vis transmittance spectra of the cured films with different ω values. | ||
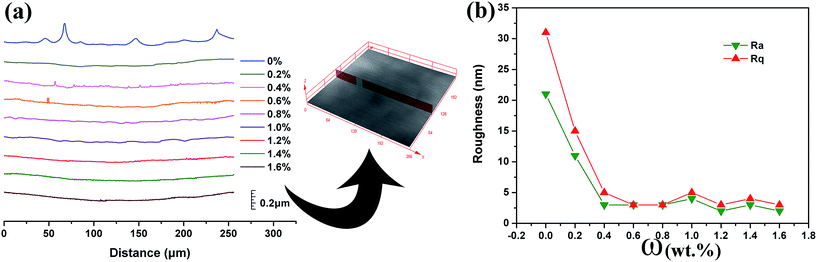
The two-dimensional surface profile curves of the cured films with different ω values are shown in Fig. 8a. It was found that the cured film without polypyrrole exhibits a rough surface with a few occasional bulges, while the other cured films appear to be smooth and uniform. In order to obtain a quantitative comparison, the average peak-to-trough height (Ra) and the square average peak-to-trough height (Rq) of the cured films were recorded using LSCM. Six random profiles were selected and the test values are averaged for each cured film, as shown in Fig. 8b. It was found that the addition of polypyrrole slightly reduces the roughness of the films. The film without polypyrrole exhibits Ra and Rq of 21 and 31 nm, respectively. A small amount of polypyrrole (ω of 0.2 and 0.4 wt%) plays an obvious role in improving the roughness of the cured films, which leads to a smaller Ra of 11 and 3 nm, respectively. However, a further increase in the polypyrrole content fails to persistently reduce the roughness of the cured films and small variations of Ra and Rq are observed in the range of 2–5 nm. It is proposed that the surface roughness of the cured film is related to Marangoni instability, which is induced by a composition or a temperature variation along the free liquid surface. Due to the fast curing rate, the liquid film without polypyrrole has insufficient time to level the Marangoni instabilities before the liquid film is fully cured, leaving a rough surface. However, the curing rate of the liquid films is slowed down with the increase of the polypyrrole loading as discussed above, resulting in an extension in the leveling time for the Marangoni instabilities compared to that of the liquid film without polypyrrole. Therefore, the surface roughness of the cured films is weakened slightly.
3.3 Efficient electrically induced structure formation of the leaky dielectric photoresist
On the basis of the above investigations, the leaky dielectric photoresist with a ω value of 1.2 wt% was selected to carry out EISF, first using a featureless template. Fig. 9a schematically displays the experimental setup of EISF onto a featureless electrode for the formation of the thin leaky dielectric photoresist film. Onto a Si substrate, the leaky dielectric photoresist film with certain thickness (h) was spin-coated, to create one electrode. Opposing it, a transparent ITO-glass electrode was placed at a certain distance (H) leaving a small air gap (H–h). For easy detemplating, the upper electrode was treated in a 1.0 wt% FAS isopropanol solution for 3 h and was subsequently baked at 150 °C for 10 h. Then a DC voltage supplied by a function/arbitrary-waveform generator (AGILENTER 33220A) was applied across the electrode pair, during which time the leaky dielectric photoresist film could flow into the peaks until liquid cylindrical bridges spanned the capacitor gap. The pattern formed during this period was cured by UV light. Finally, the top electrode was removed and a typical EISF pattern on the featureless electrode was obtained. The morphology of the EISF pattern is shown in Fig. 9b–e. It was found that there are regions of local hexagonal close-packed ordering, but no long range order because the initial fluctuations excited by thermal fluctuations on the film are distributed randomly.15 Moreover, pillar arrays with λ of 382 μm were formed within 1 s. The use of the leaky dielectric photoresist with a ω value of 1.2 wt% significantly reduces the patterning time compared to earlier approaches.14,27 This might be attributed to its low viscosity (about 5 mPa s). Furthermore, when the film is a leaky dielectric film, the accumulation of the free charges at the polymeric film–air interface result in a reduction of the electric field inside the polymeric film until the electric field reaches zero. As a consequence, the leaky dielectric film suffers a larger destabilizing electrostatic force at the polymeric film–air interface than the corresponding perfect dielectric film, which results in a further reduction in the patterning time. It is worth noting that there would be no additional reduction in the patterning time with a further increase of material conductivity when the ratio between the time scale for free charge conduction and the process time scale is extremely large.To characterize the conversion of the wetting properties of the film with its surface morphology, the static contact angles of DI water droplets on a flat film and a patterned film were measured as shown in Fig. 10. The results indicate that the intrinsic contact angle (θ) of the flat film is about 64°, while the apparent contact angle (θ*) of the patterned film reaches about 109°, which is already in the hydrophobic range. This is ascribed to the fact that the rough surface features of the patterned films have more air bubbles trapped below DI water than in the flat films, thereby resulting in the conversion of hydrophilicity into hydrophobicity.56–59 It is worth noting that λ of the pillar arrays highly depend on some external condition, such as the applied voltage, the thin film thickness, the air gap, and so on. Thus a series of micro-/nanoscale patterns with different hydrophobicities (even superhydrophobicity) can be obtained on a featureless template by altering these external conditions.
 | ||
| Fig. 10 Optical microscope images of DI water droplets (∼10 μL) on (a) a flat film and (b) a patterned film. | ||
4. Conclusions
Leaky dielectric photoresists were designed and prepared by doping a soluble conductive polypyrrole into BPA4EODMA. It is found that the leaky dielectric photoresists are homogeneous and stable in both the liquid state and the solid state owing to the low molecular weight and bulky side groups of the polypyrrole. The leaky dielectric photoresists are Newtonian fluids with low viscosities in the range of 4–7 mPa s. They display a significant increase in their electrical conductivity from ∼10−2 μS cm−1 to ∼10 μS cm−1 with an increase in the polypyrrole loading, and show favorable wettability on a silicon substrate with a contact angle of 29–38°. Meanwhile, the UV-curing process monitored using FTIR spectroscopy shows that the leaky dielectric photoresists are completely cured in 50 s. The cured film is transparent, thermally stable and featureless. Moreover, the use of the leaky dielectric photoresist with a ω value of 1.2 wt% forms pillar arrays within 10 s at ambient temperature. In addition, the contact angle of DI water on this patterned film increases noticeably from 64° to 109° in comparison to the corresponding flat film, indicating a conversion from hydrophilicity to hydrophobicity. The combination of fast photocurable behavior, Newtonian fluid properties, low viscosity, and leaky dielectric properties enables the rapid fabrication of micro-/nanostructures with small feature sizes over large areas at ambient temperature. This makes this type of leaky dielectric photoresist ideal for further applications via EISF on a featureless template.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
The research was financially supported by NSFC under Grant No. 51473133 and by the China National 973 Program under Grant No. 2009CB 724202.References
- E. Schaffer, T. Thurn-Albrecht, T. P. Russell and U. Steiner, Nature, 2000, 403, 874–877 Search PubMed.
- E. Schaffer, T. Thurn-Albrecht, T. P. Russell and U. Steiner, Europhys. Lett., 2001, 53, 518–524 Search PubMed.
- S. Harkema and U. Steiner, Adv. Funct. Mater., 2005, 15, 2016–2020 CrossRef CAS.
- X. Lei, L. Wu, P. Deshpande, Z. Yu, W. Wu, H. Ge and S. Y. Chou, Nanotechnology, 2003, 14, 786–790 CrossRef CAS.
- N. E. Voicu, M. S. M. Saifullah, K. R. V. Subramanian, M. E. Welland and U. Steiner, Soft Matter, 2007, 3, 554 RSC.
- N. E. Voicu, S. Ludwigs and U. Steiner, Adv. Mater., 2008, 20, 3022–3027 CrossRef CAS.
- Y. Zhou, A. Nicolas, K. R. Thomas and U. Steiner, Soft Matter, 2012, 8, 3841 RSC.
- X. Li, H. Tian, Y. Ding, J. Shao and Y. Wei, ACS Appl. Mater. Interfaces, 2013, 5, 9975–9982 CAS.
- H. M. Tian, Y. C. Ding, J. Y. Shao, X. M. Li and H. Z. Liu, Soft Matter, 2013, 9, 8033–8040 RSC.
- P. Goldberg-Oppenheimer, D. Kabra, S. Vignolini, S. Hüttner, M. Sommer, K. Neumann, M. Thelakkat and U. Steiner, Chem. Mater., 2013, 25, 1063–1070 CrossRef CAS.
- S. Lee, S. H. Jung, D. J. Kang and J. Lee, RSC Adv., 2016, 6, 5944–5948 RSC.
- H. Tian, J. Shao, Y. Ding, X. Li and H. Liu, RSC Adv., 2014, 4, 21672 RSC.
- H. Li, W. Yu, Y. Wang, H. Bu, Z. Liu, E. Abraham and M. P. Y. Desmulliez, RSC Adv., 2014, 4, 13774 RSC.
- M. D. Dickey, E. Collister, A. Raines, P. Tsiartas, T. Holcombe, S. V. Sreenivasan, R. T. Bonnecaze and C. G. Willson, Chem. Mater., 2006, 18, 2043–2049 CrossRef CAS.
- N. Wu and W. B. Russel, Nano Today, 2009, 4, 180–192 CrossRef CAS.
- Y. Lee, S. H. Park, K. B. Kim and J. K. Lee, Adv. Mater., 2007, 19, 2330–2335 CrossRef CAS.
- Y.-K. Lai, Z. Chen and C.-J. Lin, J. Nanoeng. Nanomanuf., 2011, 1, 18–34 CrossRef CAS.
- D. Chen, W. Zhao and T. P. Russell, ACS Nano, 2012, 6, 1479–1485 CrossRef CAS PubMed.
- S. Falah Toosi, S. Moradi, M. Ebrahimi and S. G. Hatzikiriakos, Appl. Surf. Sci., 2016, 378, 426–434 CrossRef CAS.
- Y. Liu, S. Li, S. Niu, X. Cao, Z. Han and L. Ren, Appl. Surf. Sci., 2016, 379, 230–237 CrossRef CAS.
- H. Wu, K. Zhu, B. Wu, J. Lou, Z. Zhang and G. Chai, Appl. Surf. Sci., 2016, 382, 111–120 CrossRef CAS.
- D. C. Hyun, RSC Adv., 2015, 5, 76321–76329 RSC.
- N. Wu, L. F. Pease and W. B. Russel, Adv. Funct. Mater., 2006, 16, 1992–1999 CrossRef CAS.
- N. E. Voicu, S. Harkema and U. Steiner, Adv. Funct. Mater., 2006, 16, 926–934 CrossRef CAS.
- H. Xiang, Y. Lin and T. P. Russell, Macromolecules, 2004, 37, 5358–5363 CrossRef CAS.
- H. Hu, H. Tian, X. Li, J. Shao, Y. Ding, H. Liu and N. An, ACS Appl. Mater. Interfaces, 2014, 6, 14167–14173 CAS.
- P. Goldberg-Oppenheimer and U. Steiner, Small, 2010, 6, 1248–1254 CrossRef CAS PubMed.
- L. F. Pease 3rd and W. B. Russel, J. Non-Newtonian Fluid Mech., 2002, 102, 233–250 CrossRef.
- L. F. Pease 3rd and W. B. Russel, J. Chem. Phys., 2003, 118, 3790 CrossRef.
- L. F. Pease 3rd and W. B. Russel, J. Chem. Phys., 2006, 125, 184716 CrossRef PubMed.
- L. F. Pease 3rd and W. B. Russel, Langmuir, 2004, 20, 795–804 CrossRef.
- P. Gambhire and R. M. Thaokar, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2012, 86, 036301 CrossRef CAS PubMed.
- P. Gambhire and R. M. Thaokar, Eur. Phys. J. E, 2011, 34, 84 CrossRef CAS PubMed.
- Z. Lin, T. Kerle, S. M. Baker, D. A. Hoagland, E. Schäffer, U. Steiner and T. P. Russell, J. Chem. Phys., 2001, 114, 2377 CrossRef CAS.
- Z. Lin, T. Kerle, T. P. Russell, E. Schaffer and U. Steiner, Macromolecules, 2002, 35, 3971–3976 CrossRef CAS.
- D. H. Kho, S. H. Chae, U. Jeong, H. Y. Kim and J. K. Kim, Macromolecules, 2005, 38, 3820–3827 CrossRef CAS.
- J. Bae, E. Glogowski, S. Gupta, W. Chen, T. Emrick and T. P. Russell, Macromolecules, 2008, 41, 2722–2726 CrossRef CAS.
- J. J. Rickard, I. Farrer and P. Goldberg Oppenheimer, ACS Nano, 2016, 10, 3865–3870 CrossRef CAS PubMed.
- Z. Ye, H. Cui, X. Yang and F. Qiu, J. Mater. Chem. C, 2015, 3, 1949–1956 RSC.
- J. Hwang, J. Jang, K. Hong, K. N. Kim, J. H. Han, K. Shin and C. E. Park, Carbon, 2011, 49, 106–110 CrossRef CAS.
- Z.-M. Dang, L. Wang, Y. Yin, Q. Zhang and Q.-Q. Lei, Adv. Mater., 2007, 19, 852–857 CrossRef CAS.
- C. Min, D. M. Yu, J. Y. Cao, G. L. Wang and L. H. Feng, Carbon, 2013, 55, 116–125 CrossRef CAS.
- C. E. Corcione, F. Freuli and A. Maffezzoli, Polym. Eng. Sci., 2013, 53, 531–539 CAS.
- M. Martin-Gallego, M. M. Bernal, M. Hernandez, R. Verdejo and M. A. Lopez-Manchado, Eur. Polym. J., 2013, 49, 1347–1353 CrossRef CAS.
- J. Bae, J. Ind. Eng. Chem., 2012, 18, 378–383 CrossRef CAS.
- S. Zhang, G. Wang, G. Lv, D. Yu and Y. Ding, Synth. Met., 2014, 195, 185–192 CrossRef CAS.
- W. Wang, D. Yu and F. Tian, Synth. Met., 2008, 158, 717–721 CrossRef CAS.
- R. Gangopadhyay, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 2443–2455 CrossRef CAS.
- R. Gangopadhyay, J. Colloid Interface Sci., 2009, 338, 435–443 CrossRef CAS PubMed.
- R. H. Colby, Rheol. Acta, 2009, 49, 425–442 CrossRef.
- P. E. Rouse, J. Chem. Phys., 1953, 21, 1272 CrossRef CAS.
- B. H. Zimm, J. Chem. Phys., 1956, 24, 269 CrossRef CAS.
- K. C. Tam and C. Tid, J. Rheol., 1989, 33, 257–280 CrossRef CAS.
- D.-d. Yang, H.-p. Xu, J.-r. Wang and Y.-h. Wu, J. Appl. Polym. Sci., 2013, 130, 3746–3752 CrossRef CAS.
- M. J. Uddin, T. R. Middya and B. K. Chaudhuri, Curr. Appl. Phys., 2013, 13, 461–466 CrossRef.
- S.-M. Lee and T. H. Kwon, J. Micromech. Microeng., 2007, 17, 687–692 CrossRef CAS.
- C. K. Kang, S. M. Lee, I. D. Jung, P. G. Jung, S. J. Hwang and J. S. Ko, J. Micromech. Microeng., 2008, 18, 075007 CrossRef.
- S. M. Lee, I. D. Jung and J. S. Ko, J. Micromech. Microeng., 2008, 18, 125007 CrossRef.
- C. H. Lee, P. G. Jung, S. M. Lee, S. H. Park, B. S. Shin, J.-H. Kim, K.-Y. Hwang, K. M. Kim and J. S. Ko, J. Micromech. Microeng., 2010, 20, 035018 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |