Solid state conversion of a double helix thallium(I) coordination polymer to a corrugated tape silver(I) polymer†
Maryam Moeiniana,
Kamran Akhbari*a,
Satoshi Kawatab and
Ryuta Ishikawa b
b
aSchool of Chemistry, College of Science, University of Tehran, P.O. Box 14155-6455, Tehran, Islamic Republic of Iran. E-mail: akhbari.k@khayam.ut.ac.ir; Fax: +98 21 66495291; Tel: +98 21 61113734
bDepartment of Chemistry, Fukuoka University, 8-19-1 Nanakuma, Jonan-ku, Fukuoka, 814-0180, Japan
First published on 15th August 2016
Abstract
We observed the solid state conversion of a nanostructured TlI coordination polymer with a double helix chain structure, prepared by a sonochemical procedure, to a nanostructured corrugated tape silver(I) polymer via the mechanochemical reaction of [Tl(μ2-dcpa)]n (1) [Hdcpa = 2,4-dichlorophenoxyacetic acid] with AgNO3. The internal packing of these two compounds looked similar and low-energy structural changes allowed the conversion to occur smoothly. The transformation was irreversible as a result of the formation of stronger Ag–O bonds (in 2) compared with the initial Tl–O bonds (in 1). There are weak interaction planes in the crystal packing of 1, so the structure is not mechanically rigid, which allows Ag ions to penetrate the lattice and form stronger bonds.
There has been much recent interest in coordination polymers (CPs) or metal–organic frameworks composed of metal ions and bridging ligands because of their wide range of structures and functions.1 The synthesis of thallium(I) coordination polymers is an increasingly active area of research as a result of the presence of a 6s2 electron configuration and the stereoactivity of the valence shell lone pair of electrons. Based on the directed ligands, these polymers are classified as holodirected or hemidirected.2 TlI usually favours the formation of Tl⋯Tl, Tl⋯C, Tl⋯H and Tl⋯X (X = halogen atoms) secondary interactions, especially on its vacant coordination site with a stereochemically active lone pair of electrons, which suggests that the TlI ion can act as either a Lewis acid or a Lewis base.2,3 Investigations of the structural transformations of CPs in the solid state will promote our understanding of supramolecular architectures.4 If we do not consider the stereochemical activity of the lone pair of electrons in TlI, then thallium(I) compounds are similar in structure and chemical properties to silver(I) compounds, which form Ag⋯Ag, Ag⋯C, Ag⋯H and Ag⋯X (X = halogen atoms) secondary interactions; this will facilitate the study of their structural transformation in the solid state.3,5 Structural transformations provide a promising approach to the design and synthesis of novel CPs because they give important clues about the response of CPs to external stimuli, such as changes in temperature, mechanical force, solution, reaction time, the presence of guest molecules and electromagnetic irradiation.6 CPs that show a reversible structural transformation and a characteristic response towards specific external stimuli have important applications in sensing, molecular capture and switches.6 Solid state reactions that take place under manual or mechanical grinding with minimal or no solvent have attracted attention in molecular synthesis.7 Mechanochemistry, a burgeoning field in CPs, has been used to synthesize various CPs from reactants without solvents or using liquid-assisted grinding.8 Mechanically assisted transformations offer unique advantages compared with conventional synthetic methods, such as shorter reaction times and reductions in cost, energy use and waste products, in addition to high yields relative to traditional synthesis in the liquid phase.9 We studied the mechanochemical conversion of the nanostructured [Tl(μ2-dcpa)]n (1) coordination polymer to [Ag2(μ2-dcpa)2]n (2) (Hdcpa = 2,4-dichlorophenoxyacetic acid).10 The reversibility of this solid state conversion was also studied.
The reaction between Hdcpa, KOH and TlNO3 in mixtures of methanol and distilled water provided a crystalline material with the general formula [Tl(μ2-dcpa)]n (1). Determination of the structure of compound 1 by X-ray crystallography (Tables S1 and S2†) showed the complex to be a novel one-dimensional polymer with double parallel helix chains (Fig. 1). The Tl(I) ion has a low coordination number of 2 with a TlO2 coordination sphere (Fig. S2†). The only atom coordinated with dcpa in 2 is one of the carboxylate oxygen atoms, which bridges between two TlI ions (Fig. 2 and S2†). The 2-chloro substituent and phenoxy oxygen atom of dcpa− do not link to any thallium atom (Fig. 2 and S2†). In compound 1, the lone pair of electrons in Tl(I) is active in the solid state and the arrangement of O atoms suggests a gap or hole in the coordination geometry around the Tl(I) coordination sphere (Fig. S2†), a gap possibly occupied by a stereoactive lone pair of electrons. Hence the geometry of the nearest coordination environment of every Tl(I) atom is likely to be a result of the geometrical constraints of the coordinated O atoms and to be influenced by a stereochemically active lone pair of electrons. The stereoactive lone pair of electrons in 1 is one of the factors that leads to the formation of the double helix chains.
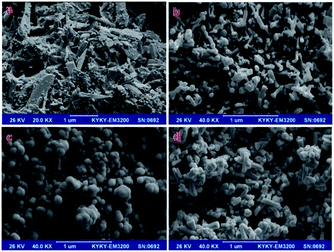
Our research also showed that the Tl in 1 is not involved in Tl⋯C, Tl⋯Tl and Tl⋯H interactions. To synthesis nanostructures of 1, Hdcpa, KOH and TlNO3 were reacted in an ultrasonic bath to give a white powder, which was then dried at room temperature. A comparison of the XRD patterns simulated from single-crystal X-ray data of 1 (Fig. S1a†) and that of the prepared powder (Fig. S1b†) confirmed the successful formation of 1. The SEM image (Fig. 3a) of the obtained powder shows that nanostructures (a mixture of nanoparticles, nanorods and nanosheets) of compound 1 were formed under ultrasonic irradiation.
In a similar manner to TlI, AgI usually favours the formation of M⋯M, M⋯C and M⋯H secondary interactions.3,5 Thus we studied the structural changes of 1 during its conversion to an Ag(I) complex of dcpa− (2) by the liquid-assisted mechanochemical reaction of 1 with excess AgNO3. The XRD pattern of 1 after a solid state mechanochemical reaction with excess AgNO3 (Fig. S1c†) showed TlNO3 in the resulting mixture, which confirmed that an ion-exchange process between Tl(I) and Ag(I) had occurred. The presence of TlNO3 in the mixture resulted in weakening and covering of the peaks of 2 in the XRD pattern, but, after the removal of TlNO3 from the mixture of products, the pure phase of 2 (Fig. S1e†) was separated. The SEM image of the mixture obtained from the mechanochemical reaction of 1 with excess AgNO3 showed the formation of other nanostructures (a mixture of nanorods and nanoparticles) (Fig. 3b) different from those seen in the precursor (Fig. 3a). After washing the prepared mixture with water, we observed a pure phase of compound 2 with a nanoparticle morphology (Fig. 3c).
Comparison of the XRD patterns simulated from the single-crystal X-ray data of the Ag(I) complex of dcpa− (2)10 (Fig. S1d†) and that of the prepared nanoparticles (Fig. S1e†) confirmed that the nanoparticles had the same structure as 2, a one-dimensional polymer with corrugated tape chains (Fig. 1 and S2†). Two types of Ag(I) ions exist in 2. The Ag(1) ion has a low coordination number of 2 with an AgO2 coordination sphere (Fig. 2). It has an approximately linear coordination with a bond angle of 174.13(18)°. The Ag(2) ion is chelated with two –COO− groups and has an AgO4 coordination sphere (Fig. 2). Against dcpa− in 1, it shows bridging and chelating behaviour in 2 (Fig. 2). The 2-chloro substituent and phenoxy oxygen atom of dcpa− do not link to any silver atom.
The Tl atom is coordinated with two oxygen atoms of two dcpa− ligands and compound 1 has an O(2)–Tl(1)–O(2) bond angle of 74.7(3)° (Fig. S2 and Table S2†). This small bond angle is a result of the stereoactive lone pair of electrons on the Tl(I) ion, which does not allow Tl(I) to form other coordination bonds. During the replacement of the Tl(I) ion with Ag(I), a linear coordination sphere around the Ag(1) and Ag(2) ions was formed with a distorted square planer structure (Fig. 2) as a result of the absence of a stereoactive lone pair of electrons on the Ag(I) ion. The internal packing of these two compounds looks similar and low-energy structural changes result in a smooth change.
The XRD pattern of 2 after the mechanochemical reaction of 2 with TlNO3 (Fig. S1f†) shows a mixture of compound 2 (with low intensity peaks marked by *) and TlNO3 (with high-intensity peaks characterized by its crystallographic planes). The absence of peaks of AgNO3 and compound 1 in the XRD pattern (Fig. S1f†) indicates that an ion-exchange process between AgI and TlI did not occur. Thus this transformation is irreversible (Fig. 1), which is a result of the formation of stronger Ag–O bonds (in 2) compared with the initial Tl–O bonds in 1.2 Because hard acids tend to bond to hard bases and AgI ion is harder than TlI ion, the tendency of hard O− ions to form a coordination bond with the AgI ion is greater than with the TlI ion, thus AgI ions (in 2) cannot be replaced by TlI ions and the conversion of 2 to 1 is impossible. There are weak interaction planes in the crystal packing of 1, so the structure is not mechanically rigid and is able to allow Ag ions to penetrate the lattice and form stronger bonds. The SEM image of the sample prepared via the reverse reaction (Fig. 3d) indicates the formation of nanorods and nanoparticles similar to those obtained from mechanochemical reaction of 1 with AgNO3 (Fig. 3b). TlNO3 thus prefers to form a nanorod morphology (compare Fig. 3b and d). By contrast, 1 tends to form nanoparticles.
The solid state structural conversion of [Tl(μ2-dcpa)]n (1) with a double helix chain structure and nanostructure morphology to a corrugated tape AgI coordination polymer [Ag2(μ2-dcpa)2]n (2) with nanoparticle morphology was observed after the mechanochemical reaction of 1 with AgNO3. The internal packing of these two compounds appears to be similar and low-energy structural changes allow the transformation to occur smoothly. The Tl atom in 1 is coordinated with the two oxygen atoms of the two dcpa− ligands and it has an O(2)–Tl(1)–O(2) bond angle of 74.7(3)°. This low bond angle is a result of a stereoactive lone pair of electrons on the Tl(I) ion, which does not allow Tl(I) to form other coordination bonds. During the replacement of the Tl(I) ion with Ag(I), a linear coordination sphere around the Ag(1) and Ag(2) ions with a distorted square planer structure was formed as a result of the absence of a stereoactive lone pair of electrons on Ag(I) ion. This transformation was irreversible because of the formation of stronger Ag–O bonds (in 2) compared with the initial Tl–O bonds (in 1). There are weak interaction planes in the crystal packing of 1, so the structure is not mechanically rigid and allows Ag ions to penetrate and form stronger bonds.
Acknowledgements
The authors acknowledge the financial support of the University of Tehran under grant number 01/1/389845 and support of this investigation by the Iran National Science Foundation.Notes and references
- M. Yoon, K. Suh, S. Natarajan and K. Kim, Angew. Chem., Int. Ed., 2013, 52, 2688 CrossRef CAS PubMed; T. Yamada, K. Otsubo, R. Makiura and H. Kitagawa, Chem. Soc. Rev., 2013, 42, 6655 RSC; S. Horike, W. Chen, T. Itakura, M. Inukai, D. Umeyama, H. Asakurae and S. Kitagawa, Chem. Commun., 2014, 50, 10241 RSC; F. Shahangi Shirazi and K. Akhbari, Inorg. Chim. Acta, 2015, 436, 1 CrossRef; Z.-J. Li, S. K. Khani, K. Akhbari, A. Morsali and P. Retailleau, Microporous Mesoporous Mater., 2014, 199, 93 CrossRef; K. Akhbari, A. Morsali and M. Zeller, J. Organomet. Chem., 2007, 692, 3788 CrossRef.
- K. Akhbari and A. Morsali, Coord. Chem. Rev., 2010, 254, 1977 CrossRef CAS.
- K. Akhbari and A. Morsali, CrystEngComm, 2012, 14, 1618 RSC; K. Akhbari and A. Morsali, J. Organomet. Chem., 2007, 692, 5109 CrossRef CAS; K. Akhbari and A. Morsali, J. Organomet. Chem., 2007, 692, 5141 CrossRef; K. Akhbari and A. Morsali, Inorg. Chem. Commun., 2007, 10, 1189 CrossRef; K. Akhbari, A. Morsali, A. D. Hunter and M. Zeller, Inorg. Chem. Commun., 2007, 10, 178 CrossRef.
- S. Kitagawa and K. Uemura, Chem. Soc. Rev., 2005, 34, 109 RSC; F. Shahangi Shirazi and K. Akhbari, RSC Adv., 2015, 5, 50778 RSC; K. Akhbari and A. Morsali, Inorg. Chem., 2013, 52, 2787 CrossRef CAS PubMed.
- S. Hunig, H. Meixner, T. Metzenthin, U. Langohr, J. U. Schutz, H.-C. Wolf and E. Tillmanns, Adv. Mater., 1990, 2, 361 CrossRef CAS; K. Akhbari, A. Morsali and P. Retailleau, Polyhedron, 2010, 29, 3304 CrossRef; K. Akhbari and A. Morsali, Inorg. Chim. Acta, 2010, 363, 1435 CrossRef; S. Zheng, M. Tong, S. Tan, Y. Wang, J. Shi, Y. Tong, H. Lee and X. Chen, Organometallics, 2001, 20, 5319 CrossRef; J. Zhang, Y. Lin, X. Huang and X. Chen, Inorg. Chem., 2005, 44, 3146 CrossRef PubMed.
- X.-Y. Yi, H.-C. Fang, Z.-G. Gu, Z.-Y. Zhou, Y.-P. Cai, J. Tian and P. K. Thallapally, Cryst. Growth Des., 2011, 11, 2824 Search PubMed; D. J. Lun, G. I. Waterhouse and S. G. Telfer, J. Am. Chem. Soc., 2011, 133, 5806 CrossRef CAS PubMed; M. Nagarathinam, A. Chanthapally, S. H. Lapidus, P. W. Stephens and J. J. Vittal, Chem. Commun., 2012, 48, 2585 RSC; Y.-Q. Lan, H.-L. Jiang, S.-L. Li and Q. Xu, Inorg. Chem., 2012, 51, 7484 CrossRef PubMed; Y. S. Tan, A. L. Sudlow, K. C. Molloy, Y. Morishima, K. Fujisawa, W. J. Jackson, W. Henderson, S. N. B. A. Halim, S. W. Ng and E. R. Tiekink, Cryst. Growth Des., 2013, 13, 3046 Search PubMed; D. Yuan, D. Zhao, D. J. Timmons and H.-C. Zhou, Chem. Sci., 2011, 2, 103 RSC; M. Kang, G.-P. Yang, L. Hou, W.-P. Wu, Y.-L. Wu and Y.-Y. Wang, CrystEngComm, 2015, 17, 1839 RSC.
- D. Braga, S. L. Giaffreda, F. Grepioni, A. Pettersen, L. Maini, M. Curzi and M. Polito, Dalton Trans., 2006, 1249 RSC; A. L. Garay, A. Pichon and S. L. James, Chem. Soc. Rev., 2007, 36, 846 RSC; T. Friščić, J. Mater. Chem., 2010, 20, 7599 RSC.
- V. Andre, A. Hardeman, I. Halasz, R. S. Stein, G. J. Jackson, D. G. Reid, M. J. Duer, C. Curfs, M. T. Duarte and T. Friščić, Angew. Chem., Int. Ed., 2011, 50, 7858 CrossRef CAS PubMed; V. Strukil, L. Fabian, D. G. Reid, M. J. Duer, G. J. Jackson, M. Eckert-Maksic and T. Friščić, Chem. Commun., 2010, 46, 9191 RSC.
- A. Briceño, D. Leal and G. D. de Delgado, New J. Chem., 2015, 39, 4965 RSC.
- T. C. W. Mak, W.-H. Yip, C. H. L. Kennard, G. Smith and E. J. O'Reilly, J. Chem. Soc., Dalton Trans., 1988, 2353 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, XRPD patterns, TGA curves and other figure. CCDC 1438353 (1). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra09423f |
| This journal is © The Royal Society of Chemistry 2016 |