Predicting surface tension for vegetable oil and biodiesel fuels
Abstract
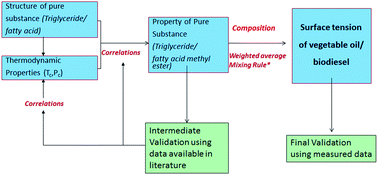
Vegetable oil and biodiesel are considered as alternatives to diesel fuel due to their favorable engine characteristics and renewable nature. Estimates of their surface tension values are essential in understanding fuel spray behavior. This study proposes an approach for predicting the surface tension of vegetable oil and biodiesel based on their composition. In the proposed methodology, the surface tension of fatty acids and methyl esters are first estimated using suitable property correlations available in the literature. The suitability of correlations is adjudged based on validation with the measured data. Further, the correlations are also modified to improve the predictions. A weighted average mixing rule is then employed to determine the surface tension of the vegetable oil and biodiesel from their measured composition. The predicted and measured surface tension values of karanja, palmolein and coconut are compared and found to agree within 7 percent over a useful temperature range of up to 353 K. The effects of transesterification and compositional variations on the surface tension of biodiesel fuels are also discussed in this paper.


 Please wait while we load your content...
Please wait while we load your content...