Facile synthesis and enhanced visible-light photoactivity of a g-C3N4/mullite composite
Chunquan Li,
Zhiming Sun*,
Lixin Liu,
Weixin Huang and
Shuilin Zheng
School of Chemical and Environmental Engineering, China University of Mining and Technology (Beijing), Beijing 100083, P. R. China. E-mail: zhimingsun@cumtb.edu.cn; Fax: +86 10 62339920
First published on 13th September 2016
Abstract
A novel g-C3N4/mullite composite with enhanced visible light-driven photoactivity was prepared through a facile wetting chemical method. The microstructure, interfacial and optical properties of the obtained g-C3N4/mullite composites were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FTIR), surface area measurement (BET), and UV-visible diffused reflectance spectroscopy (UV-vis DRS), respectively. It is indicated that a tight interfacial combination was formed between g-C3N4 and mullite, which is beneficial for the transfer of electrons and the enhancement of quantum efficiency. Compared with pure g-C3N4 or a g-C3N4 + mullite physical mixture, the synthesized g-C3N4/mullite composites exhibit significantly enhanced photoactivity under visible light irradiation, almost 6.5 times that of the pure g-C3N4 and around 10.0 times that of a g-C3N4 + mullite physical mixture for the degradation of tetracycline. The enhanced photoactivity of the g-C3N4/mullite composite could be attributed not only to its stronger visible light adsorption and enhanced adsorption capacity for pollutants but also to the strong interfacial combination between g-C3N4 and mullite, effectively reducing the recombination probability of photogenerated electron–hole pairs.
1. Introduction
Over the past decades, tetracycline (TC) has been widely used in aquiculture and the livestock industry. As one typical and extensively utilized antibiotic, tetracycline in the environment derives from direct runoff and excretion by human beings and animals which is not completely metabolized.1 Recently, tetracycline residues in the aquatic environment have become one of the most concerning issues considering their potential adverse effects on human beings and the aquatic ecosystem. Tetracycline could increase the resistance of bacteria, have genotoxic effects on microorganisms and thus threaten human health.2 Hence, it is imperative to develop efficient technologies for eliminating tetracycline from water. In recent years, various techniques have been proposed to treat tetracycline contaminated water, including adsorption,3 membrane filtration,4 biodegradation2 and photocatalysis.5 Among these methods, photocatalysis with high activity and good stability seems to be the most suitable and attractive way to deal with the tetracycline contaminated water.Among various photocatalysts, considerable attention has been focused on the environmental applications of graphitic carbon nitride (g-C3N4) photocatalyst since it was first applied to the photocatalytic water splitting under visible-light irradiation.6 The g-C3N4 has been proved to be a novel organic semiconductor due to its visible light responsive band gap (2.7 eV), reliable chemical inertness and stability. Nevertheless, similar to the other semiconductors with narrow band gap, the g-C3N4 photocatalyst suffers fast charge recombination and slow charge transport. To overcome the above-mentioned drawbacks, numerous strategies have been explored to enhance the quantum efficiency of g-C3N4, such as nonmetal or metal doping,7,8 noble metals deposition,9,10 porous structure fabrication,11 and heterojunction construction.12,13 However, other drawbacks such as easy agglomeration, difficult separation and low adsorption ability could not be well solved through such modifications. Synthesis of composite photocatalysts using natural minerals as catalyst carriers seems to be one ideal way to solve those practical application problems of g-C3N4 photocatalyst.14–16 Many previous literature reports have mentioned that the highly efficient photocatalysts would be obtained through incorporating g-C3N4 with Al2O3 or SiO2 due to the defect sites on the surface, which would regulate the surface structure and facilitate the transfer of carriers.17–23 Mullite, as a solid solution which derived from the calcination of the natural layered kaolinite over 1100 °C, is a typical kind of Al2O3–SiO2 binary system.24 Thus, the mullite seems to be a promising candidate as g-C3N4 sheet supports due to its thermal, mechanical and optical properties. To the best of our knowledge, the photocatalytic effect of mullite combining with g-C3N4 sheet has not been reported yet.
In the current work, a novel kind of g-C3N4/mullite composite with strong interfacial interaction was synthesized through facile wetting chemical method. The physicochemical properties of as-received composites were characterized by different instruments. The photocatalytic performance of g-C3N4/mullite composites was evaluated by the photodegradation of tetracycline in aqueous solution under visible light. The influences of g-C3N4 amounts in composites and solution pH on the visible-light driven photocatalytic efficiency were investigated. In addition, the reusability of the composite photocatalyst was examined. The enhanced photocatalytic activities for tetracycline as well as the enhancement mechanism were also discussed based on the experimental results.
2. Experimental
2.1. Materials
The mullite was obtained from Huasheng kaolin Co. Ltd. (Inner Mongolia, China). Tetracycline hydrochloride (TC, C22H24N2O8·HCl, USP Grade) was obtained from Amresco Biosciences. Dicyandiamide, edetate disodium (EDTA-2Na), benzoquinone (BQ), t-butyl alcohol (t-BuOH), hydrochloric acid (HCl), sodium hydrate (NaOH) and other chemicals used in our experiments were purchased from Beijing Reagent Co. (Beijing, China). All other chemicals were analytical reagent grade and used without any further purification. The deionized water was used throughout this study.2.2. Preparation of g-C3N4/mullite composites
The fabrication of g-C3N4 powders followed the below procedure. Typically, 15 g of dicyandiamide was put into an alumina crucible with a cover, and heated to 550 °C for 4 h with a heating rate of 2.3 °C min−1. Then, the sample was further heated at 500 °C for 2 h. After natural cooling to room temperature, the final resulted yellow product was collected and ground into powder for further use.The g-C3N4/mullite composites were synthesized by a facile process. Firstly, various amounts of dicyandiamide were added to 50 mL of deionized water, and dissolved by magnetic stirring in a water bath for 15 min at 60 °C. In the following step, 2 g of mullite powder was put into above solution and immersing for 12 h under magnetic stirring at 60 °C. Then, the product was collected and dried at 60 °C for 12 h. After simple grinding, the g-C3N4/mullite composites were calcined following the same fabrication conditions of g-C3N4 powders. The g-C3N4/mullite composites with different mass ratios of g-C3N4 with respect to mullite were synthesized and labeled as g-C3N4/mullite-1, g-C3N4/mullite-2, g-C3N4/mullite-3, g-C3N4/mullite-4 and g-C3N4/mullite-5, respectively. According to the thermogravimetric analysis (TG) results, the content of g-C3N4 in the composites can be calculated as 6.87%, 16.29%, 23.59%, 30.00% and 46.34%, respectively. For comparison, the g-C3N4/mullite physical mixture (g-C3N4 + mullite) was also prepared as follows: 2 g of mullite powder and 0.86 g of pure g-C3N4 were added to 50 mL deionized water, and then mixed for 12 h through magnetic stirring in a water bath at 60 °C. The final product was dried in an oven at 60 °C for 12 h, and it was named as g-C3N4 + mullite.
2.3. Characterizations
The thermogravimetric analysis (TG) were carried out on a METTLER SF/1382 thermal analyzer at a heating rate of 10 °C min−1 under a O2 atmosphere from room temperature to 1000 °C. X-ray diffraction (XRD) were performed on a D8 advance X-ray diffractometer (Bruker, Germany) equipped with Cu-Kα radiation (λ = 0.154056 nm) to identify the crystalline phase of the obtained photocatalysts. The samples were scanned from 10° to 80° with a 0.02° step at a scanning speed of 4° min−1. An S-4800 scanning electron microscopy (Hitachi, Japan) was applied to investigate the surface morphology and constitute of samples. Transmission electron microscope (TEM) of samples was performed on a Hitachi HT-7700 operating at 100 kV. The surface area of samples were measured by N2 adsorption at 77 K on a constant volume adsorption apparatus (JW-BK, JWGB Sci. & Tech., China) and calculated by the Brunaer–Emmett–Teller (BET) method. Fourier-transform infrared spectroscopy (FTIR) was measured on a Nicolet 6700 spectrometer using conventional KBr pellets. The optical properties of the as-received samples were studied by UV-vis diffuse reflectance spectroscopy (DRS) using a UV-vis spectrophotometer (U-3010, Hitachi), where BaSO4 was used as the reference. The band gap values of different photocatalysts were estimated by extrapolating the linear part of the plot of (F(R)hν)1/2 versus hν: F(R)hν = A(hν − Eg)2, where F(R) = (1 − R)2/2R stands for the Kubelka–Munk function calculated from the reflectance spectrum and hν is the photon energy expressed in eV. PL spectra of the catalysts were measured on the F-7000 spectrometer (Hitachi, Japan) with an excitation wavelength of 370 nm. The photocurrent and electrochemical impedance spectroscopy (EIS) were measured with an electrochemical analyzer (CHI-660B, China).2.4. Photoactivity measurements
The photocatalytic activities of as-prepared g-C3N4/mullite composites were evaluated by the degradation of tetracycline (TC) under a 500 W xenon lamp (PL-03, Beijing Pulinsaisi plant, China) with a 400 nm cut-off filter. In a typical experiment, 0.2 g of the as-prepared catalysts was suspended in 100 mL of standard TC (20 ppm) aqueous solution. Prior to illumination, the suspension was magnetically stirred in the dark for 1 h to achieve the adsorption–desorption equilibrium on the surface of materials. At a given time interval, 2 mL of suspension was sampled and separated through centrifugation at 8000 rpm for 5 min. Photodegradation effect was determined by measuring the absorbance of the solution at 359 nm on a UV-vis spectrophotometer. Comparative experiments were carried out under the same conditions using pure g-C3N4, mullite, and g-C3N4 + mullite physical mixture as references. The influences of g-C3N4 amounts in composites and solution pH on photocatalytic efficiency were also investigated in our work. Besides, the stability of the composite photocatalyst was examined by reusability test. All the removal data of batch experiments were obtained in parallel.2.5. Photoelectrochemical measurement
To investigate the photoelectrochemical properties of the as-prepared samples, the working electrodes were prepared as follows: 10 mg of the prepared photocatalyst was suspended in 1 mL ethanol and ultrasonicated for 15 min to generate the slurry. Then, the resulting suspension was dropped onto the ITO slice with a fixed area of 2 cm2 and dried in air. Finally, it was transferred to the oven for 4 h at 80 °C. All the photoelectrochemical measurements were conducted on an electrochemical analyzer in a standard three-electrode system using the prepared samples as the working electrode, a Pt wire as the counter electrode, and Ag/AgCl as a reference electrode. A 500 W Xe arc lamp served as the light source. The photocurrent and electrochemical impedance spectroscopy (EIS) was performed in 0.1 M Na2SO4 aqueous solution.3. Results and discussion
3.1. Characterizations
XRD patterns of the mullite, pure g-C3N4 and g-C3N4/mullite composites are presented in Fig. 1. The XRD pattern of mullite exhibits several peaks at 16.42°, 26.22°, 33.19°, 35.19°, 36.94° and 60.62°, which are well indexed to the orthorhombic phase of mullite (JCPDS no. 50-1249). As for pure g-C3N4, two obvious peaks at 12.8° and 27.8° can be attributed to the (100) and (002) diffraction planes of the graphic carbon nitride, which corresponds to the interlayer structural packing arrangement parallel to the c-axis and the long-range interplanar stacking of the conjugated aromatic system.25 As for the g-C3N4/mullite composites, it is noticed that the intensity of the characteristic peaks of mullite decreases with increasing the g-C3N4 dosage, which was attributed to the constitution variation of the composites and the formation of intimate interfacial combination during the calcination process after incorporating the g-C3N4.24 The peak intensity of g-C3N4/mullite composites at 12.8° and 27.8° strengthens with increasing the amount of g-C3N4. Besides, the (002) diffraction peak of the g-C3N4/mullite composites shifts to the higher angle compared with the pure g-C3N4, implying the interaction effect existed between the mullite and g-C3N4 nanosheets in the composites.26To investigate the microstructures of mullite, g-C3N4 and g-C3N4/mullite composite, the SEM and TEM measurements were conducted in the study. As shown in Fig. 2(a), it is indicated that the mullite has an irregular structure with relatively smooth surface. According to Fig. 2(b), the pure g-C3N4 displays lamellar structures stacking with each other, which would not only decreases the absorption ability of g-C3N4 but also accelerates the velocity of electron–hole pairs' recombination. Compared with the mullite, the surface of as-prepared g-C3N4/mullite composite becomes rougher as shown in Fig. 2(c) and (d). Furthermore, the g-C3N4 nanosheets were densely and evenly decorated on the surface of mullite. The enhanced dispersity of g-C3N4 nanosheets would favor the transfer of the photo electrons and facilitate the improvement of quantum efficiency. Fig. 2(e) and (f) shows the TEM images of g-C3N4/mullite composite. It is confirmed that the tight combination between pure g-C3N4 and mullite was constructed. This obtained intimated interfacial contact between g-C3N4 and mullite would be beneficial for the transfer of electrons to the surface of mullite, which could provide a lot of surface vacancy or defect sites to induce the separation of photogenerated electrons and holes.18,21 In conclusion, SEM and TEM observations confirm the successful synthesis of g-C3N4/mullite composite.
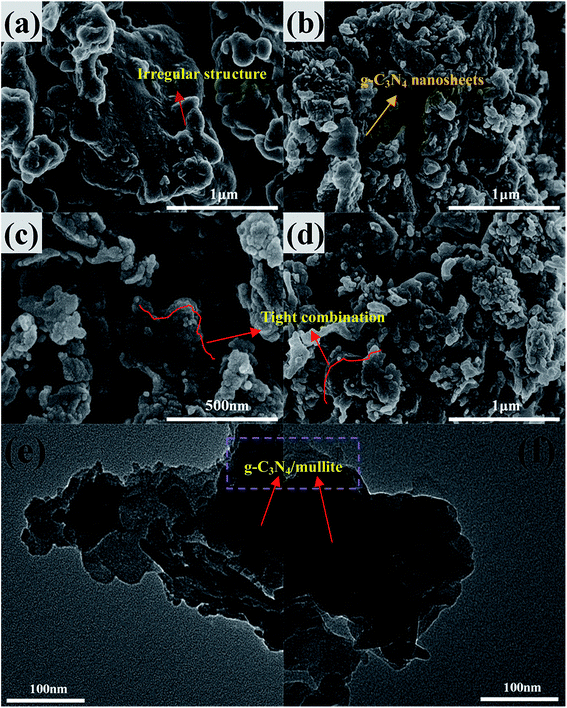 | ||
| Fig. 2 FESEM images of (a) mullite, (b) g-C3N4 and (c and d) g-C3N4/mullite-4, TEM images of (e and f) g-C3N4/mullite-4. | ||
N2 adsorption–desorption isotherms and BJH pore size distributions of g-C3N4, mullite and g-C3N4/mullite composite are shown in Fig. 3. The specific surface area (SBET), pore volume (VP), as well as the average adsorption and desorption pore size of g-C3N4, mullite and the as-prepared g-C3N4/mullite composites are summarized in Table 1. From Fig. 3(a), it is observed that the mullite and g-C3N4/mullite-4 show a type II adsorption isotherm according to the International Union of Pure and Applied Chemistry (IUPAC), which indicated the presence of macropore in the mullite. As for pure g-C3N4, it is observed that pure g-C3N4 exhibits a typical type IV adsorption isotherm and the H3 hysteresis loop, which proved the lamellar structure of pure g-C3N4.27 The pore size of the as-received g-C3N4/mullite composite is 2–10 nm according to the Fig. 3(b), which might contribute to the adsorption of contaminant molecules in water.28 According to Table 1, the surface areas and pore volumes of the g-C3N4/mullite composites are improved as compared with those of mullite and g-C3N4. With increasing the g-C3N4 content, the specific surface area of composites decreased first and then increased. It is indicated that combining the pure g-C3N4 with mullite would be favorable for the formation of higher specific surface areas composites, which are beneficial for pollutant adsorption and providing more surface active sites for pollutant degradation.29
 | ||
| Fig. 3 (a) N2 adsorption and desorption isotherms measured at 77 K; (b) BJH pore size distribution plots. | ||
| Sample | SBET (m2 g−1) | Pore volume (cm3 g−1) | Average pore radius (nm) |
|---|---|---|---|
| Mullite | 6.755 | 0.017 | 9.950 |
| g-C3N4/mullite-1 | 27.470 | 0.038 | 5.563 |
| g-C3N4/mullite-2 | 17.936 | 0.040 | 8.809 |
| g-C3N4/mullite-3 | 14.308 | 0.035 | 9.655 |
| g-C3N4/mullite-4 | 20.917 | 0.042 | 8.013 |
| g-C3N4/mullite-5 | 24.640 | 0.047 | 7.659 |
| g-C3N4 | 15.316 | 0.043 | 11.175 |
FTIR analysis was also conducted to investigate the composition and interfacial structure of the synthesized composite. As seen from Fig. 4(a), the bands at 562 and 892 cm−1 could be well assigned to the standard orthorhombic mullite which was in agreement with XRD analysis. Besides, the intensity of the adsorption band at 469 and 1099 cm−1 could be attributed to Si–O–Si vibration while the band at 741 cm−1 could be ascribed to Al–O–Al vibration, which indicated that silicon and aluminum elements emerged in the formation of oxides in the mullite.30,31 As for the pristine g-C3N4, the strong band of 1200–1700 cm−1, with the characteristic peaks at 1239, 1321, 1405, 1575, and 1638 cm−1, was assigned to the typical stretching vibration of CN heterocycles (Fig. 4(b)). In addition, the peak at 808 cm−1 was well corresponding to the breathing mode of triazine units.32 When it was referred to the g-C3N4/mullite composite, around 2 cm−1 red shift could be noticed at the peaks of 1239, 1321, 1405, 1575 and 1638 cm−1. It is also noticed that the vibration intensity of CN was obvious enhanced. Both of them proved that there is an interfacial interaction between g-C3N4 and mullite, which would facilitate the transfer of photoinduced carriers and improve the photocatalytic activity of the g-C3N4/mullite composite.33
 | ||
| Fig. 4 FTIR spectra of mullite, g-C3N4 and as-prepared g-C3N4/mullite composite (a) and the magnified FTIR spectra of g-C3N4/mullite composite in the range from 1150 cm−1 to 1750 cm−1 (b). | ||
The optical absorption property of photocatalyst is another key factor determining its photocatalytic performance.34 To investigate the optical absorption properties of the mullite and the as-prepared photocatalysts, the UV-vis optical absorption spectra was also collected as shown in Fig. 5(a). It is clear that the pure g-C3N4 shows strong absorption with the adsorption edge at around 460 nm and an extended tail to the region near 600 nm. The mullite exhibits weaker light-adsorption ability within the scope of full spectrum. Compared with pure g-C3N4 and mullite, the received g-C3N4/mullite composites show better absorption ability from UV light to visible light. In addition, the absorption edge of the as-received g-C3N4/mullite composites had a red shift to the visible light zone and the absorption intensity in the range of full spectrum is obviously increased in comparison with that of pure g-C3N4, which could be attributed to the diffuse scattering effect derived from the combination and the interfacial interaction effect between g-C3N4 and mullite. The band gaps of mullite, g-C3N4 and g-C3N4/mullite composites were calculated and displayed in Fig. 5(b). The band gap of pure g-C3N4 is determined to be 2.74 eV, which is in agreement with previous report.35 The proper band gaps of g-C3N4/mullite composites in the range of 2.64–3.00 eV would enhance the visible light absorption ability, which might be beneficial for the generation of more photogenerated carriers under visible light irradiation.
 | ||
| Fig. 5 UV-vis DRS (a) and band gaps (b) of mullite, g-C3N4 and as-prepared g-C3N4/mullite composites. | ||
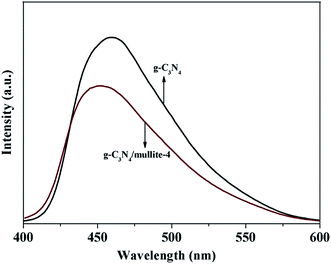
To give further evidence to confirm the synergistic effect of mullite and g-C3N4, photoluminescence (PL) spectra was performed to reveal the charge transfer, migration and recombination processes of the pure g-C3N4 and g-C3N4/mullite composite (Fig. 6). The PL spectra of g-C3N4/mullite composite shows a blue shift of about 10 nm compared with pure g-C3N4 owing to the quantum confinement effect.36 It is indicated that the emission band for pure g-C3N4 is located at around 460 nm, while the PL intensity of the g-C3N4/mullite composite decreases compared with the pure g-C3N4. This might be attributed to the improved interfacial charge transfer because of the synergistic effect of mullite and g-C3N4, which plays an important role in reducing the recombination between electrons and holes.
In order to further understand the photogenerated charge separation and electron transfer performance of the as-prepared photocatalyst, the transient photocurrent responses of pristine mullite, g-C3N4 and g-C3N4/mullite composite were recorded for several on–off cycles under visible light irradiation (Fig. 7(a)). Notably, the g-C3N4/mullite composite exhibits an enhanced photocurrent response than that of pure mullite and g-C3N4, which indicated that a greatly improved charge separation was obtained through the establishment of tight interfaces. Furthermore, electrochemical impedance spectroscopy (EIS) was also conducted and the result was shown in Fig. 7(b). The radius of the arc on the EIS Nyquist plot reveals the charge transfer rate at the contact interface between the working electrode and electrolyte solution. Generally, the smaller radius of the Nyquist circle represents the lower charge transfer resistance. It is clear that the arc radius of g-C3N4/mullite composite is smaller than that of pure mullite and g-C3N4, which indicated the fastest separation and transfer rate of electrons and holes in the interfaces.
 | ||
| Fig. 7 (a) Transient photocurrent response and (b) Nyquist plots of EIS obtained for g-C3N4, mullite and g-C3N4/mullite composite. | ||
3.2. Photocatalytic activity
The photocatalytic activities of g-C3N4/mullite composites were mainly evaluated by photocatalytic degradation of TC aqueous solution under visible light irradiation (λ > 400 nm). The TC degradation curves of the photocatalysts and linear transform ln(C0/C) of the kinetic curves under visible light are shown in Fig. 8. For comparison, the removal effects of mullite, g-C3N4, TC self-decomposition, and g-C3N4 + mullite physical mixture were also determined as references under the same conditions. Prior to illumination, all the samples experienced 1 h in dark to reach the equilibrium of adsorption and desorption. As shown in Fig. 8(a), it is obvious that the photoinduced self-decomposition of TC can be negligible. Although the single mullite and pure g-C3N4 has poor adsorption ability towards TC, the g-C3N4/mullite composites showed enhanced adsorption capacity due to their larger surface areas compared with the mullite and pure g-C3N4. It is noticed that about 20–37% of TC has been absorbed by g-C3N4/mullite composites in the dark, while the amount of TC absorbed by pure g-C3N4 is only 4%. The concentration of TC gradually decreased as the exposure time increased for all samples except for the single mullite. It is indicated that the pure g-C3N4, g-C3N4/mullite composites and g-C3N4 + mullite mixture exhibited visible light responsive photoactivities. The pure g-C3N4 and g-C3N4 + mullite mixture showed poorer photocatalytic activities compared with g-C3N4/mullite composites. When the g-C3N4 respect to mullite was up to 30.00% (g-C3N4/mullite-4), the g-C3N4/mullite composites displayed the optimal photocatalytic activity. The final removal rate of g-C3N4/mullite-4 reached to 92%, which are almost 3 times that of pure g-C3N4 and around 4 times that of g-C3N4 + mullite physical mixture. From above data, it is obvious that the high degradation activity of g-C3N4/mullite composites is not just attributed to the adsorption capability. Combined with the previous characterization analysis results, it is proposed that the enhanced photocatalytic activity of g-C3N4/mullite composites should be ascribed to the high dispersity of g-C3N4 on the surface of mullite and the high migration efficiency of photo-induced electrons.37 When the bulk g-C3N4 was densely decorated on the surface of mullite, it is favorable for TC molecular to absorb on the g-C3N4 nanosheets. The ultrathin thickness could significantly shorten the diffuse distance and time of photogenerated carriers, which promote interfacial photocatalytic redox reactions.26 | ||
| Fig. 8 (a) Photocatalytic degradation of and (b) linear transform ln(C0/C) of the kinetic curves of TC under visible light. | ||
To further quantitatively evaluate the photocatalytic abilities of the various g-C3N4/mullite composites, the pseudo-first-order reaction constant is taken using the following equation:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0/C = kt + ln C0/C = kt + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0/C1 C0/C1
| (1) |
| Samples | Pseudo-first order | ||
|---|---|---|---|
| Fitted equation | k (min−1) | Correlation coefficient (R2) | |
| g-C3N4 | y = 0.00093x + 0.0191 | 0.00093 | 0.9384 |
| g-C3N4/mullite-1 | y = 0.00078x − 0.0173 | 0.00078 | 0.9803 |
| g-C3N4/mullite-2 | y = 0.00207x + 0.0127 | 0.00207 | 0.9890 |
| g-C3N4/mullite-3 | y = 0.00466x + 0.0477 | 0.00466 | 0.9912 |
| g-C3N4/mullite-4 | y = 0.00602x + 0.0985 | 0.00602 | 0.9928 |
| g-C3N4/mullite-5 | y = 0.00434x + 0.0314 | 0.00434 | 0.9942 |
| g-C3N4 + mullite | y = 0.00062x + 0.0255 | 0.00062 | 0.9741 |
The effects of solution pH on the photodegradation of TC in the presence of g-C3N4/mullite-4 composite were also conducted in this study. Different pH values of 3, 5, 7, 9, and 11 were selected according to the previous report.5 According to Fig. 9, it is observed that 90% of TC was photodegraded at pH 7, which is higher than acid or alkali conditions. Moreover, compared with alkali condition, the photodegradation rate of composite is decreased under acid condition, which indicated that the generation of reactive oxygen species would be more inhibited under acid condition.38
The reusability experimental results of g-C3N4/mullite-4 are illustrated in Fig. 10. As shown in Fig. 10, although a 2.5% reduction appeared within 6 h under visible light after experiencing the fourth cycle, the as-received photocatalyst still remains a high degradation rate after four reaction cycles, which means the as-synthesized g-C3N4/mullite photocatalyst shows good reusability. The slight reduction might be ascribed to intermediate poison to the surface of the photocatalyst, which could decline the adsorption capacity as well as the electron transfer velocity.39
3.3. Possible mechanism
To further investigate the main active species accounting for the photocatalytic degradation, scavenger experiments were conducted in the presence of EDTA-2Na, BQ and t-BuOH as scavengers for holes, ˙O2− and ˙OH radicals, respectively. The photocatalytic degradation efficiencies over g-C3N4/mullite composite under the visible light are displayed in Fig. 11. Without the addition of the scavengers, the photocatalytic degradation rate of TC was up to around 91% after 6 h irradiation. On the other hand, the photocatalytic degradation efficiency was decreased to various extent after the addition of three scavengers, which indicated that holes, ˙O2− and ˙OH radicals were indeed photogenerated on the surface of composites under irradiation. In comparison with the other two scavengers, the decrease of TC removal rates is the minimum in the presence of t-BuOH, indicating that ˙OH has minor effect on the degradation of TC. The photocatalytic degradation efficiencies of TC over g-C3N4/mullite composite are greatly depressed when the EDTA-2Na and BQ are added, showing both holes and ˙O2− are the main reactive species in this system. | ||
| Fig. 11 The effects of different scavengers on the degradation of TC in the presence of g-C3N4/mullite-4 composite. | ||
Based on aforementioned experimental data and analysis, the potential electrons transfer route and possible mechanism for TC degradation over g-C3N4/mullite composite are presented in Fig. 12. Under visible light illumination, electrons (e−) are easily excited from the highest occupied molecular orbital (HOMO) of g-C3N4 to its lowest unoccupied molecular orbital (LUMO), inducing the formation of holes (h+) in the HOMO. According to the previous literature reports,19,21 the surface of mullite would exist some defect or vacancy sites, which is beneficial for the electrons migration and the enhancement of photocatalytic efficiency. As illustrated in Fig. 12, the generated electrons can migrate to the defect sites as electron acceptor, enhancing the separation efficiency of photogenerated electrons and holes.18
 | ||
| Fig. 12 Schematic illustration of the charge separation and photocatalytic mechanism of g-C3N4/mullite-4 under visible light irradiation. | ||
The enhancement mechanisms of photocatalytic activity for TC degradation over the g-C3N4/mullite composites can be summarized as follows. Firstly, the g-C3N4 nanosheets were well loaded on the surface of mullite to form the tight interfacial combination through the SEM and TEM analysis, which could shorten the migration distance of the photogenerated charges and promote more carriers to participate in the photocatalytic reactions. Secondly, enlarged surface areas of the composites and enhanced adsorption capacity for pollutant would be beneficial for the transfer of reactants as well as providing more reaction active sites. The stronger visible light absorption of the composites is also beneficial for the enhancement of photocatalytic activity. Finally, the defect and vacancy sites of the mullite could trap the photogenerated electrons and then effectively reduce the recombination of photogenerated electron–hole pairs.
4. Conclusions
In summary, a novel kind of visible-light activity of g-C3N4/mullite composite was successfully synthesized via facile wetting chemical method. The XRD, BET, FTIR, UV-vis DRS and morphological analysis reveals that the g-C3N4 nanosheets were well dispersed on the surface of mullite. The as-synthesized g-C3N4/mullite-4 sample exhibited the optimal photocatalytic activity towards TC under visible light irradiation, the reaction rate constant of which is almost 6.5 times that of pure g-C3N4 and around 10.0 times that of g-C3N4 + mullite physical mixture. The neutral condition is more favorable for the TC degradation compared with the acid or alkali condition. And the g-C3N4/mullite composite shows good reusability after four cycles. The holes and ˙O2− are the main reactive species in the process of degradating TC under visible light irradiation. The enhancement in the photocatalytic performance of the composite is mainly attributed not only to its stronger visible light adsorption and enhanced adsorption capacity for pollutant but also to the enhanced quantum efficiency arising from the process of electrons transfer to the defect sites of the mullite through the strong interfacial combination between g-C3N4 and mullite, which can effectively reduce the recombination probability of photogenerated electron–hole pairs.Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (Grant No. 51504263) and the Fundamental Research Funds for the Central Universities (2015QH01).References
- J. Niu, S. Ding, L. Zhang, J. Zhao and C. Feng, Chemosphere, 2013, 93, 1–8 CrossRef CAS PubMed.
- V. Maroga Mboula, V. Hequet, Y. Gru, R. Colin and Y. Andres, J. Hazard. Mater., 2012, 209–210, 355–364 CrossRef CAS PubMed.
- M. E. Parolo, M. C. Savini, J. M. Vallés, M. T. Baschini and M. J. Avena, Appl. Clay Sci., 2008, 40, 179–186 CrossRef CAS.
- C. M. Zhang, D. Cong, H. Xu, Y. H. Miao, Y. Y. Cheng, T. Hao, J. H. Zhou and X. C. Wang, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2015, 50, 744–749 CrossRef CAS PubMed.
- P. Wang, P.-S. Yap and T.-T. Lim, Appl. Catal., A, 2011, 399, 252–261 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed.
- X. Wang, X. Chen, A. Thomas, X. Fu and M. Antonietti, Adv. Mater., 2009, 21, 1609–1612 CrossRef CAS.
- Y. Bu, Z. Chen and W. Li, Appl. Catal., B, 2014, 144, 622–630 CrossRef CAS.
- T. Han, X. Li, Y. Li, W. Cao, D. Wu, B. Du and Q. Wei, Sens. Actuators, B, 2014, 205, 176–183 CrossRef CAS.
- X. Chen, Y. S. Jun, K. Takanabe, K. Maeda, K. Domen, X. Fu, M. Antonietti and X. Wang, Chem. Mater., 2009, 21, 4093–4095 CrossRef CAS.
- J. X. Sun, Y. P. Yuan, L. G. Qiu, X. Jiang, A. J. Xie, Y. H. Shen and J. F. Zhu, Dalton Trans., 2012, 41, 6756–6763 RSC.
- F. Shi, L. Chen, M. Chen and D. Jiang, Chem. Commun., 2015, 51, 17144–17147 RSC.
- Z. Sun, C. Bai, S. Zheng, X. Yang and R. L. Frost, Appl. Catal., A, 2013, 458, 103–110 CrossRef CAS.
- Q. Sun, X. Hu, S. Zheng, Z. Sun, S. Liu and H. Li, Powder Technol., 2015, 274, 88–97 CrossRef CAS.
- B. Wang, G. Zhang, Z. Sun and S. Zheng, Powder Technol., 2014, 262, 1–8 CrossRef CAS.
- F. T. Li, S. J. Liu, Y. B. Xue, X. J. Wang, Y. J. Hao, J. Zhao, R. H. Liu and D. Zhao, Chemistry, 2015, 21, 10149–10159 CrossRef CAS PubMed.
- F. T. Li, Y. Zhao, Q. Wang, X. J. Wang, Y. J. Hao, R. H. Liu and D. Zhao, J. Hazard. Mater., 2015, 283, 371–381 CrossRef CAS PubMed.
- F.-T. Li, Y.-B. Xue, B. Li, Y.-J. Hao, X.-J. Wang, R.-H. Liu and J. Zhao, Ind. Eng. Chem. Res., 2014, 141205091652003, DOI:10.1021/ie5036258.
- J. Chen, S. Shen, P. Guo, M. Wang, J. Su, D. Zhao and L. Guo, J. Mater. Res., 2013, 29, 64–70 CrossRef.
- B. Lin, C. Xue, X. Yan, G. Yang, G. Yang and B. Yang, Appl. Surf. Sci., 2015, 357, 346–355 CrossRef CAS.
- M. Shalom, S. Inal, D. Neher and M. Antonietti, Catal. Today, 2014, 225, 185–190 CrossRef CAS.
- X. Wang, S. Wang, W. Hu, J. Cai, L. Zhang, L. Dong, L. Zhao and Y. He, Mater. Lett., 2014, 115, 53–56 CrossRef CAS.
- E. Ruiz de Sola, F. J. Serrano, E. Delgado-Pinar, M. M. Reventós, A. I. Pardo, M. A. Kojdecki, J. M. Amigó and J. Alarcón, J. Eur. Ceram. Soc., 2007, 27, 2647–2654 CrossRef CAS.
- X. Wang, X. Chen, A. Thomas, X. Fu and M. Antonietti, Adv. Mater., 2009, 21, 1609–1612 CrossRef CAS.
- J. Li, E. Liu, Y. Ma, X. Hu, J. Wan, L. Sun and J. Fan, Appl. Surf. Sci., 2016, 364, 694–702 CrossRef CAS.
- K. S. W. Sing, Pure Appl. Chem., 2009, 57, 603–619 Search PubMed.
- Z. Wu, H. Joo, I. S. Ahn, S. Haam, J. H. Kim and K. Lee, Chem. Eng. J., 2004, 102, 277–282 CrossRef CAS.
- Y. Li, J. Zhan, L. Huang, H. Xu, H. Li, R. Zhang and S. Wu, RSC Adv., 2014, 4, 11831–11839 RSC.
- S. Sembiring, W. Simanjuntak, P. Manurung, D. Asmi and I. M. Low, Ceram. Int., 2014, 40, 7067–7072 CrossRef CAS.
- H. M. Zhou, X. C. Qiao and J. G. Yu, Appl. Clay Sci., 2013, 80–81, 176–181 CrossRef CAS.
- M. Xu, L. Han and S. Dong, ACS Appl. Mater. Interfaces, 2013, 5, 12533–12540 CAS.
- D. Jiang, J. Li, C. Xing, Z. Zhang, S. Meng and M. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 19234–19242 CAS.
- T. Junwang, Z. Zhigang and Y. Jinhua, Angew. Chem., Int. Ed., 2004, 43, 4463–4466 CrossRef PubMed.
- Z. Zhang, D. Jiang, D. Li, M. He and M. Chen, Appl. Catal., B, 2016, 183, 113–123 CrossRef CAS.
- Z. Xiaodong, X. Xiao, W. Hui, Z. Jiajia, P. Bicai and X. Yi, J. Am. Chem. Soc., 2012, 135, 18–21 Search PubMed.
- J. Ma, Q. Liu, L. Zhu, J. Zou, K. Wang, M. Yang and S. Komarneni, Appl. Catal., B, 2016, 182, 26–32 CrossRef CAS.
- Y. Chen, C. Hu, J. Qu and M. Yang, J. Photochem. Photobiol., A, 2008, 197, 81–87 CrossRef CAS.
- S. Liu, H. Sun, A. Suvorova and S. Wang, Chem. Eng. J., 2013, 229, 533–539 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |