Curing kinetics, thermal and mechanical properties of TDE-85 modified by bicyclo-benzoxazine†
Abstract
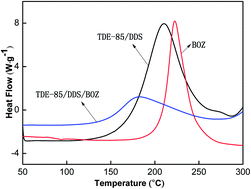
To reduce the curing temperature and also to enhance the mechanical properties and the heat resistance of diglycidyl-4,5-epoxy-cyclohexane-1,2-dicarboxylate (TDE-85), it was crosslinked with 4,4-diamino diphenyl sulphone (DDS) using a synthesized bicyclo-benzoxazine (BOZ) crosslinker. The kinetic parameters of the curing reaction were evaluated using Flynn–Wall–Ozawa and Kissinger's methods. The gelation time and differential scanning calorimetry (DSC) of non-isothermal testing were performed to determine the curing process of BOZ/DDS/TDE-85 systems. Fourier transform infrared spectroscopy (FTIR) was used to follow the major changes in functional groups during the curing process. The absorbance of the oxazine ring showed a significant reduction and the peak of epoxy group became very weak, indicating that the curing reaction was almost complete. The thermal properties were evaluated by heat distortion temperature (HDT) and thermogravimetric analysis (TGA), exhibiting a higher initial decomposition temperature, decreased rate of decomposition and higher char yield compared to the neat epoxy resin system. BOZ enhanced the stability of the epoxy blend, which restricted the mobility of the chain and hindered the decomposition process. The fractured surface of the cured product was observed by scanning electron microscopy (SEM). The fracture form of BOZ/DDS/TDE-85 systems belongs to typically ductile fracture compared to the neat DDS/TDE-85 system. It was found that the thermal and mechanical properties of BOZ/DDS/TDE-85 systems increased with the addition of a certain amount of BOZ. The impact, flexural, and compressive strengths increased by 43.4%, 13.1%, and 8.5%, respectively. The addition of BOZ lowered the activation energy on account of the reduced viscosity, allowing better contact of the resin with the curing agent. The reaction order and activation energy were found to be 0.92 and 63.15 kJ mol−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...