Largely improved electromechanical properties of thermoplastic dielectric elastomers by grafting carboxyl onto SBS through thiol–ene click chemistry
Ming Tian*abc,
Haichao Yanac,
Haibin Sunac,
Liqun Zhangabc and
Nanying Ning*abc
aKey Laboratory of Beijing City on Preparation and Processing of Novel Polymer Materials, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: Tianm@mail.buct.edu.cn
bKey Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029, China
cState Key Laboratory of Organic-Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, China
First published on 22nd September 2016
Abstract
In this study, we report an approach to graft carboxyl groups onto the polybutadiene (PB) domains of a styrene–butadiene–styrene triblock copolymer (SBS) through thiol–ene click chemistry to prepare a homogeneous thermoplastic dielectric elastomer with largely improved electromechanical properties. The grafting degree of the carboxyl groups dipoles on SBS can be controlled by irradiation time to control the polarizability and thus the electromechanical properties of SBS. The tensile strength of modified SBS significantly decreases due to the improved miscibility of polystyrene (PS) and PB domains but still greater than 1 MPa. Importantly, the dielectric constant (εr) at 1000 Hz largely increased from 2.2 for pristine SBS to 7.2 for modified SBS with the grafting degree of 83%, ascribed to the increase in the polarizability of modified SBS by grafting the carboxyl groups on the PB block. The maximum actuated strains increased to 1.75% for modified SBS with a grafting degree of 52%, which is almost a 4-fold increase over that of pristine SBS (0.47%). This study provides us with a simple, effective and controllable method to synthesise homogeneous thermoplastic dielectric elastomers with largely improved dielectric constants and actuated strain.
1 Introduction
Dielectric elastomers (DEs) are one of the electro-active polymers (EAPs) that can respond to a certain electric field by changing their shape. Because of their excellent property of light weight, large strain, fast response, reliability, high energy density, and high efficiency, DEs have potential to be used as actuators for applications in robotic manipulators,1–3 artificial muscles,4 sensors5 and energy recovery.6,7 A dielectric elastomer actuator (DEAs) is composed of an elastomer film sandwiched between two thin and compliant electrodes. When voltage is applied the film can expand in the plane and decrease in the thickness direction.The strain in thickness direction is given by:
 | (1) |
Up to now, many studies have been done to improve the actuated strain based on the eqn (1). Up to now, a commonly used approach to increase the β is to introduce high εr ceramics such as BaTiO3 (ref. 9 and 10), TiO2 (ref. 11 and 12) or inorganic conductive fillers such as carbon nanotubes,13 graphene14 or conductive polymers polyaniline (PANI)15 and poly(hexylthiophene)16 into the elastomer matrix to prepare the DE composites or DE blends. Due to the high dielectric constant of the filler and the increase of interfacial polarization, the εr of the composites can be greatly enhanced. However, there are some disadvantages for DE composites. First, the fillers especially the inorganic fillers always exhibit high Young's modulus which could decrease the actuated strain at certain voltage and lead to the deteriorated processability.17,18 Secondly, the good interfacial interaction and the homogeneous dispersion of these fillers in elastomer are hard to be achieved because of the heterogeneous nature between the fillers and the matrix, thus the stress concentration and interface debonding could occur during actuation, leading to the decrease in breakdown strength of the DE composites. What's more, the introduction of the fillers especially the conductive fillers usually leads to the increase in dielectric loss.11,19
Some studies were focused on decreasing the Y of DE and improving the strain sensitivity through introducing plasticizers into elastomer matrix.11,20 However, the introduction of the plasticizer often sacrifices the mechanical properties and the durability of the actuators. Meanwhile, the plasticizer molecules volatile or migrating nature limits the application of the DEs.
Another widely used method to improve the actuated strain of DEs is to prepare new homogeneous DE materials with high permittivity and high breakdown strength by chemical synthesis or graft-modification.21–24 Some previous studies reported that the new structured homogeneous DE prepared by grafting high permittivity organic polymers or oligomers onto elastomer chains exhibited obviously enhanced permittivity with almost no reduction in breakdown strength. Meanwhile, the electromechanical response and the maximum actuation strain of these new structured homogeneous DEs was achieved.25 In other studies, the new homogeneous DEs were prepared by grafting functionalized dipoles onto elastomer chains, and the results showed that the new structured DEs exhibited largely improved permittivity and actuated strain at a given electrical field.26
The thiol–ene click reaction is extremely rapid, well-controlled and copper-free reaction under mild reaction conditions and has pervasive application in the synthesis of dendritic molecules,24 crosslinking materials,25 modified compounds27 and so on. In this study, we used a thermoplastic elastomer, styrene–butadiene–styrene triblock copolymer (SBS) as DE because of its good mechanical strength, easy processability (vulcanization is not required), low energy consumption and recyclability. We grafted the carboxyl groups with strong polarity onto the SBS by using the simple photochemical thiol–ene click reaction to increase the εr and actuated strain of DEs. Our goal is to design and prepare homogeneous “green” DEs with enhanced dielectric constant and actuated strain, good mechanical strength and easy processability by using a simple, effective and controllable chemical method.
2 Materials and methods
Poly(styrene)-block-poly(butadiene)-block-poly(styrene) triblock copolymers (SBS), thioglycolic acid (TGA) and 2,2-bimethoxy-2-phenylacetophenone (DMPA) as photoinitiator were all purchased from Sigma-Aldrich. Anhydrous tetrahydrofuran (THF), hexane were obtained from J&K Chemical Technology (Shanghai City, China). All chemicals were used as received.First, 5 g of SBS was dissolved in 50 ml THF under stirring until the SBS completely dissolved to obtain a transparent solution of 10 wt% SBS in THF. After the dissolution of SBS, 0.2 g of photoinitiator and 29.8 g of thioglycolic acid were then added into the solution under the flow of nitrogen in case of the TGA was oxidized. Then the solution was subjected to irradiation and subsequently concentration and precipitation as described in previous report.27 Finally, modified SBS films were then prepared by solution casting and dried in a vacuum at 50 °C until reaching constant mass, yielding a product with relatively low solubility in THF. The grafting degree was controlled by controlling the reacting time. To get different grafting degreed SBS, we set the reacting time as 2 min, 5 min, 15 min and 30 min.
3 Characterizations and measurements
The existence of the carboxyl groups was characterized by Fourier Transform Infrared Spectroscopy (Tensor 27, Bruker Optik GmBH, Germany) at a resolution of 4 cm−1. A minimum of 24 scans were signal averaged, and the spectra were stored on a magnetic disc system. The frequency scale is internally calibrated with a reference helium–neon laser to an accuracy of 0.01 cm−1. The films used in this study were sufficiently thin to be within an absorbance range where the Beer–Lambert law is obeyed.13C NMR spectroscopy of polymers were carried out with a Bruker AV300 spectrometery at the frequency of 12 kHz. 13C NMR integrations are calibrated to the carbon atoms of SBS, we can locate the carbon atoms in different chemical environments according to 13C NMR spectroscopy.
An atomic force microscope (AFM, Multimode 8, Nano Scope Analysis, Bruker, Germany) was used to observe the morphology of pristine and modified SBS by using the tapping mode. The samples for AFM observations were prepared by method of polishing. The AFM images were collected at a scanning rate of 1 Hz.
The stress–strain curves of pristine SBS and modified SBS were obtained by using a tensile apparatus (Instron 5567, USA) at room temperature and a crosshead speed of 50 mm min−1. Dumbbell specimens (length × width × thickness: 20 × 4 × 0.5 mm3) were used. The elastic modulus of a specimen was determined by the slope of the stress–strain curve within 10% strain.
A resistivity meter (EST 121, Beijing Huajinghui Technology Co., Ltd., China) was used to measure the volume conductivity of pristine and modified SBS according to National Standard of China GB/1410-2006. The electrical conductivity can be calculated as follows:
| σ = 1/ρv = t/RS |
The dielectric properties were measured by using a broadband dielectric spectrometer (Alpha-A, Novochtrol, Germany) in the frequency range of 102 to 106 Hz at room temperature and at the electric voltage of 3 V. The samples had a diameter of 25 mm and a thickness of 0.5 mm.
The compliant electrodes were fabricated by spraying a graphite suspension composed of graphite, silicone oil, and curing agent28 on the two main surfaces of the film with an airbrush. A circular strain test was carried out to measure the actuated strain. The dielectric elastomer film was fixed on a circle frame. The strain was defined as the change in the pixel of the electrodes' area divided by the original pixel area. The voltage was supplied by a high-voltage direct current generator (DTZH-60, Wuhan Dotek Electric Co., Ltd.). In order to obtain the change in the pixel of the electrodes' area, a video camera was fixed above the film to capture the actuator plane before and after applying the voltage with the same focal length, and then the captured video pictures were processed with Photoshop software.
4 Results and discussion
The carboxyl groups in thioglycolic acid (TGA) were grafted onto the double bonds of the SBS through thiol–ene reaction. The main reaction principle is shown in Scheme 1. The principle of the reaction is based on the free radical chain mechanism and initiated by UV light. The main reaction style may be the addition of TGA on the main chain and anti-Markovnikov addition on the side chain.27 There are also some side-reactions like Markovnikov addition on the pendant double bonds, and cyclization reaction, as show in Scheme 1a. | ||
| Scheme 1 (a) The principle of the thiol–ene click reaction between TGA and SBS and (b) schematic representation of SBS and modified SBS by thiol–ene reaction. | ||
Because of the carboxyl groups of grafted TGA behave the characteristics of acidic, we can calculate the grafting degree of carboxyl groups on SBS by means of the quantitative titration of grafted carboxyl using acid–base reaction. Meanwhile, we calculated the unreacted double bonds of SBS using the method of iodimetry. The results of the grafted carboxyl groups and the remained double bonds are fitted well. The curve of grafting degree with reaction time by quantitative titration is shown in Fig. 1. For simplicity, the final products are noted as SBS–TGA-6%, SBS–TGA-19%, SBS–TGA-52% and SBS–TGA-83% based on the grafting degree, obtained at the reaction time of 2 min, 5 min, 15 min and 30 min individually.
To verify the conversion of the thiol–ene click reaction, FTIR spectra of pristine SBS, TGA and modified SBS were recorded by potassium bromide coating method (Fig. 2). The characteristic peak of pristine SBS occurs at 966 cm−1, representing the double bonds of PB, weaker as the increase of reacting time. The characteristic peak at 2550 cm−1 of TGA, representing the S–H groups, disappears on the spectrum of modified SBS, indicating the unreacted TGA are totally removed from the modified SBS. The wide associating peak in the range of 3000–3500 cm−1 indicating the existence of OH. In the thioglycolic acid molecular there exist two bands relating to carboxyl groups: at 1740 cm−1 (monomeric form) and 1685 cm−1 (associated form).29,30 But for modified SBS, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double-bond stretching vibration appeared at 1708 cm−1 which shifted to the lower wavenumber compared with the monomeric form carboxyl groups, indicating the formation of hydrogen bonds. Besides, the appearance of characteristic peaks at 1279 cm−1 which represent the C–O stretching mode of TGA, and the decreasing of the peak at 966 cm−1 which represents the double bonds of PB both demonstrates the success grafting of carboxyl groups on SBS.
O double-bond stretching vibration appeared at 1708 cm−1 which shifted to the lower wavenumber compared with the monomeric form carboxyl groups, indicating the formation of hydrogen bonds. Besides, the appearance of characteristic peaks at 1279 cm−1 which represent the C–O stretching mode of TGA, and the decreasing of the peak at 966 cm−1 which represents the double bonds of PB both demonstrates the success grafting of carboxyl groups on SBS.
Since the solubility of modified SBS decreases in nonpolar deuterated solvent like deuterochloroform due to the introduction of polar carboxyl groups to the chains of SBS, 13C NMR rather than 1H NMR was used to confirm the variation of the molecular structure of the pristine SBS and SBS–TGA-83%. The 13C NMR spectra of pristine SBS and modified SBS are shown in Fig. 3. The chemical shifts are assigned according to the ref. 31–33. Compared to the solid state 13C NMR spectra of pristine SBS, a new peak at 175.7 ppm, which is assigned to carbonyl signal,34 is observed in SBS–TGA-83% besides the expected signals for the block copolymer. In addition, the peak at 130.1 ppm, which is assigned to the double bonds of PB domains, disappears for SBS–TGA-83%. A new peak appears at 68.1 ppm which is assigned to the –CH2– adjacent to the S atoms and the peak of tertiary carbon atoms at 27.2 ppm is intensified on the spectrum of modified SBS, demonstrating the grafting of carboxyl groups on SBS by thiol–ene reaction.
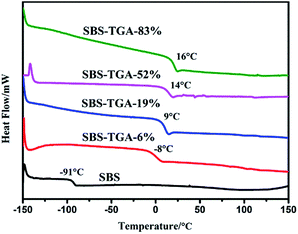
The glass transition temperature (Tg) of pristine and modified SBS were obtained by using DSC, which were previously subjected to heating above their melting temperature and further cooling to −150 °C to eliminate thermal history. As can be seen in Fig. 4, compared with the pristine SBS, the Tg of PB domain largely increases from −91 °C for pristine SBS to −8 °C, 9 °C, 14 °C and 16 °C for the modified SBS with the grafting degree of 6%, 19%, 52%, 83%, respectively. The reason is that the grafted carboxyl groups restrict the movement of PB segments. The large increase in Tg also suggests the successful thiol–ene reaction of SBS.
The phase morphology of pristine and modified SBS was observed by using AFM, and the results are shown in Fig. 5, where the bright regions represent PS phase and the darker regions represent PB phase. For pristine SBS, the incompatibility between PS and PB blocks, and the big difference in chain mobility of the two blocks results in the severe phase separation of PS and PB, and the formation of co-continuous structure of large PS domains in continuous PB phase.27 For modified SBS, the polar carboxyl groups were introduced onto the PB chains, leading to the increase in the compatibility between PB and PS domains. As we can see, phase mixing of PS and PB occurs for all the modified samples, and the size of both PB and PS phase obviously decreases with the increase in grafting degree.
 | ||
| Fig. 5 AFM phase images with 1 × 1 μm scans of (a) pristine SBS (b–d) modified SBS with 19%, 52% and 83% of grafting degree individually. | ||
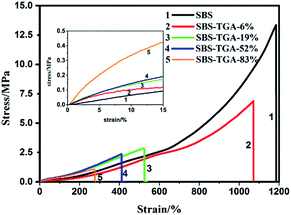
The stress–strain curves of pristine SBS and modified SBS are shown in Fig. 6, and the tensile strength and the Y of these samples are summarized in Table 1. As we can see, the pristine SBS exhibits relatively high mechanical strength up to 13.3 MPa, which is attributed to the special phase separated structure in SBS. The tensile strength of modified SBS significantly decreases due to increase in the miscibility of PS and PB domains (see the AFM morphology). However, the tensile strength of these DEs is still larger than 1 MPa, ensuring the good mechanical strength of DE. It is unusual that the elastic modulus of the modified SBS visibly increases because of the strong intermolecular interactions by hydrogen bonding of carboxyl groups in PB domains, as described above.
| Samples | Y (kPa) | Tensile strength, (MPa) | εr at 103 Hz | tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at 103 Hz δ at 103 Hz |
β at 103 Hz MPa−1 | Volume resistivity (Ω cm) | Maximum planar actuated strainb (%) | Breakdown strength (kV mm−1) |
|---|---|---|---|---|---|---|---|---|
| a The films involved in the table herein are all with no prestrain.b The conversion formula between the planar actuation strain and the actuation strain in thickness direction is according to equation: (SA + 1)(SZ + 1) = 1 in which SA is planar actuation strain and SZ is actuation strain in thickness direction. | ||||||||
| Organic dipoles grafted silicone35 | 550 | 0.83 | 5.9 | 0.014 | 10.7 | — | 1.3 | 15.3 |
| SBS | 534 | 13.3 | 2.2 | 0.005 | 4.1 | 9.3 × 1014 | 0.47 | 60 |
| SBS–TGA-52% | 1020 | 2.4 | 5.8 | 0.15 | 5.7 | 2.8 × 1011 | 1.75 | 35 |
| SBS–TGA-83% | 3340 | 1.1 | 7.2 | 0.30 | 2.2 | 4.9 × 1010 | 0.11 | 16 |
The dielectric properties versus frequency of pristine SBS and modified SBS at room temperature are presented in Fig. 7. For pristine SBS and modified SBS with a low grafting degree, the εr is almost constant in the whole frequency range from 102 to 106 Hz as shown in Fig. 7a. With the increase of grafting degree to 52% and 83%, the εr shows an obvious frequency dependence at low frequency range, ascribed to the increase in the dipole polarizability of SBS chains because of the graft of carboxyl groups on PB block. Importantly, the εr of modified SBS at the same frequency obviously increases with the increase in the grafting degree. As an example, the εr at 103 Hz of pristine SBS and modified SBS are summarized in Table 1. The εr at 103 Hz obviously increases from 2.2 for stock SBS to 5.8 and 7.2 for SBS–TGA-52% and SBS–TGA-83% individually. The increase of εr with grafting degree is ascribed to the increase in the dipole polarizability of SBS chains caused by the introduction of carboxyl groups.
The dielectric loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) of pristine SBS and modified SBS is shown in Fig. 7b. For example, we summarized the tan
δ) of pristine SBS and modified SBS is shown in Fig. 7b. For example, we summarized the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at 103 Hz of the samples in Table 1. Obviously, the tan
δ at 103 Hz of the samples in Table 1. Obviously, the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at 103 slightly increases with the increase in the graft degree of carboxyl groups on SBS, ascribed to the increase in the local current generated during dipole polarization, as indicated by the increase in electrical conductivity after modification (see Table 1).
δ at 103 slightly increases with the increase in the graft degree of carboxyl groups on SBS, ascribed to the increase in the local current generated during dipole polarization, as indicated by the increase in electrical conductivity after modification (see Table 1).
Fig. 8 presents the actuated strains of pristine SBS and modified SBS as a function of electric field. Although the breakdown strength of the modified SBS decreases, the maximum actuated strains largely increases to 1.75% for SBS–TGA-52%, which is almost 4-fold of that of pristine SBS (0.47%). It is worth noting that although the higher grafting degree (83%) of SBS exhibits the higher εr, the dielectric loss and the elastic modulus increases at the same time, and thus the β for SBS–TGA-83% even lower than that of pristine SBS, which further induces the decrease of the actuated strain. This indicates that it is better to graft appropriate amount of polar groups on SBS to increase the polarizability and ensure the moderate modulus. Compared with the reported organic dipoles grafted silicone,35 the modified SBS we get possess the virtues of the higher strength and elastic modulus, reusable, vulcanization-free and increased actuation strain.
5 Conclusions
We successfully grafted the carboxyl groups on the PB domains of SBS through thiol–ene click chemistry to prepare homogeneous thermoplastic dielectric elastomer with largely improved electromechanical properties. The grafting of carboxyl groups on SBS increases the compatibility between PB and PS block, leading to the phase mixing of modified SBS. As a result, the tensile strength of modified SBS obviously decreases but still higher than 1 MPa, superior to the modified silicone dielectric elastomer.35 The εr largely increases from 2.2 for pristine SBS to 7.2 for modified SBS for grafting degree of 83%, and the maximum actuated strain accordingly increase from 0.47% to 1.75%. This study provides a simple, effective and controllable method to synthesis homogeneous thermoplastic dielectric elastomers with largely improved dielectric constant and actuated strain.Acknowledgements
We would like to express our sincere thanks to the National Natural Science Foundation of China (Grant No. 51525301, 51473011, 51521062) for financial supports.References
- Y. B. Cohen, Smart Structures & Materials Electroactive Polymer Actuators & Devices, 2001, p. 136 Search PubMed
.
- J. Sébastien, PhD thesis, Massachusetts Institute of Technology, 2007
.
- R. Pelrine, R. D. Kornbluh, J. Eckerle, P. Jeuck, S. Oh, Q. Pei and S. Stanford, Proc. SPIE-Int. Soc. Opt. Eng., 2001, 148–156 CrossRef CAS
.
- Z. Li, L. A. Fredin, P. Tewari, S. A. DiBenedetto, M. T. Lanagan, M. A. Ratner and T. J. Marks, Chem. Mater., 2010, 22, 5154–5164 CrossRef CAS
.
- R. Trujillo, J. Mou, P. E. Phelan and D. S. Chau, Int. J. Adv. Manuf. Tech., 2004, 23, 176–182 CrossRef
.
- R. Pelrine, R. Kornbluh, Q. Pei and J. Joseph, Science, 2000, 287, 836–839 CrossRef CAS PubMed
.
- F. Carpi, S. Bauer and D. De Rossi, Science, 2010, 330, 1759–1761 CrossRef CAS PubMed
.
- R. K. Ron Pelrine, J. Joseph, R. Heydt, Q. Pei and S. Chiba, Mater. Sci. Eng., C, 2000, 11, 0928–4931 Search PubMed
.
- D. Yang, M. Tian, D. Li, W. Wang, F. Ge and L. Zhang, J. Mater. Chem. A, 2013, 1, 12276–12284 CAS
.
- H. M. Jung, J.-H. Kang, S. Y. Yang, J. C. Won and Y. S. Kim, Chem. Mater., 2010, 22, 450–456 CrossRef CAS
.
- D. Yang, L. Zhang, N. Ning, D. Li, Z. Wang, T. Nishi, K. Ito and M. Tian, RSC Adv., 2013, 3, 21896–21904 RSC
.
- H. Liu, L. Zhang, D. Yang, Y. Yu, L. Yao and M. Tian, Soft Mater., 2013, 11, 363–370 CrossRef CAS
.
- S. Liu, M. Tian, L. Zhang, Y. Lu, T. W. Chan and N. Ning, J. Mater. Sci., 2016, 51, 2616–2626 CrossRef CAS
.
- M. Tian, J. Zhang, L. Q. Zhang, S. T. Liu, X. Q. Zan, T. Nishi and N. Y. Ning, J. Colloid Interface Sci., 2014, 430, 249–256 CrossRef CAS PubMed
.
- M. Molberg, D. Crespy, P. Rupper, F. Nuesch, J. A. E. Manson, C. Lowe and D. M. Opris, Adv. Funct. Mater., 2010, 20, 3280–3291 CrossRef CAS
.
- F. Carpi, G. Gallone, F. Galantini and D. De Rossi, Adv. Funct. Mater., 2008, 18, 235–241 CrossRef CAS
.
- B. Balakrisnan and E. Smela, SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, 2010 Search PubMed
.
- G. Stiubianu, A. Bele, M. Cazacu, C. Racles, S. Vlad and M. Ignat, Mater. Res. Bull., 2015, 71, 67–74 CrossRef CAS
.
- D. Yang, RSC Adv., 2015, 5, 70500 RSC
.
- D. M. Opris, M. Molberg, C. Walder, Y. S. Ko, B. Fischer and F. A. Nüesch, Adv. Funct. Mater., 2011, 21, 3531–3539 CrossRef CAS
.
- S. Risse, B. Kussmaul, H. Krüger and G. Kofod, RSC Adv., 2012, 2, 9029–9035 RSC
.
- H. Stoyanov, M. Kollosche, D. N. McCarthy and G. Kofod, J. Mater. Chem., 2010, 20, 7558–7564 RSC
.
- C. Racles, M. Cazacu, B. Fischer and D. M. Opris, Smart Mater. Struct., 2013, 22, 104004 CrossRef
.
- S. J. Dünki, Y. S. Ko, F. A. Nüesch and D. M. Opris, Adv. Funct. Mater., 2015, 25, 2467–2475 CrossRef
.
- H. Stoyanov, M. Kollosche, D. N. McCarthy and G. Kofod, J. Mater. Chem., 2010, 20, 7558 RSC
.
- B. Kussmaul, S. Risse, G. Kofod, R. Waché, M. Wegener, D. N. McCarthy, H. Krüger and R. Gerhard, Adv. Funct. Mater., 2011, 21, 4589–4594 CrossRef CAS
.
- J. S. Silverstein, B. J. Casey, M. E. Natoli, B. J. Dair and P. Kofinas, Macromolecules, 2012, 45, 3161–3167 CrossRef CAS
.
- G. Kofod, Dielectric Elastomer Actuators, PhD thesis, Risø National Laboratory, Technical University of Denmark, 2001 Search PubMed
.
- P. E. Kireeva, G. A. Shandryuk, J. V. Kostina, G. N. Bondarenko, P. Singh, G. W. Cleary and M. M. Feldstein, J. Appl. Polym. Sci., 2007, 105, 3017–3036 CrossRef CAS
.
- M. M. Feldstein, T. I. Kiseleva, G. N. Bondarenko, J. V. Kostina, P. Singh and G. W. Cleary, J. Appl. Polym. Sci., 2009, 112, 1142–1165 CrossRef CAS
.
- H. Yu, A. Natansohn, M. A. Singh and T. Plivelíc, Macromolecules, 1999, 32, 7562–7571 CrossRef CAS
.
- R. Qi, Q. Yu, Y. Shen, Q. Liu and C. Zhou, J. Appl. Polym. Sci., 2006, 102, 5274–5279 CrossRef CAS
.
- A.-K. Du, Q. Zhou, Z.-B. Wen, J.-W. Yang, J. M. N. v. Kasteren and Y.-Z. Wang, Polym. Degrad. Stab., 2011, 96, 870–874 CrossRef CAS
.
- W. Heinen, S. W. Erkens, M. V. Duin and J. Lugtenburg, J. Polym. Sci., Part A: Polym. Chem., 1999, 4368–4385 CrossRef CAS
.
- B. Kussmaul, S. Risse, G. Kofod, R. Waché, M. Wegener, D. N. Mccarthy, H. Krüger and R. Gerhard, Adv. Funct. Mater., 2011, 21, 4589–4594 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |