Mono- and triiodophenyl isocyanate as radiopacifying agents for methacrylate-based copolymers; biocompatibility and non-toxicity
Saeed Shiralizadeh,
Hossein Nasr-Isfahani*,
Ali Keivanloo and
Mohammad Bakherad
School of Chemistry, Shahrood University of Technology, Shahrood 3619995161, Iran. E-mail: Saeed_alizadeh86@yahoo.com; akeivanloo@yahoo.com; m.bakherad@yahoo.com; Nasrisfahani@yahoo.com
First published on 7th November 2016
Abstract
New radiopaque acrylic copolymers were prepared via the copolymerization of methyl methacrylate (MMA) and acrylic acid (AA). The copolymers were made radiopaque through the reaction of carboxylic acid groups with 4-iodophenyl isocyanate and 3,4,5-triiodophenyl isocyanate moieties, as radiopacifying agents. The iodinated copolymers were characterized by Fourier transform infrared (FT-IR) spectroscopy, nuclear magnetic resonance (NMR), energy dispersive X-ray (EDAX), gel permeation chromatography (GPC), elemental analysis, differential scanning calorimetry (DSC), and thermogravimetric analysis (TGA). The composition and iodine content of the copolymers were demonstrated via acid–base and potentiometric titrations, respectively. The radiopacity of the copolymers was investigated by X-radiography. To evaluate the biocompatibility of the iodinated copolymers, a direct MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was carried out on Swiss mouse embryonic stem tissue fibroblast-like cells (NIH3T3 cell line) according to the ISO10993-5 standard. The results indicated that the new iodinated copolymers are thermally stable and have high radiopacity. It was also found that the newly radiopaque copolymers have no cytotoxicity, and could be useful for biomedical applications.
1. Introduction
Radiopacity is an essential and efficient feature that can be added to biomaterials since it facilitates the positioning and assessment of devices using the non-destructive X-radiography technique. Radiopaque materials have been designed for a variety of medical applications such as embolization processes, preparation of implants, prostheses, and tracing a drug-loaded polymer or nanoparticle in the body after a local performance. In a modern radiological diagnostic procedure, radiopaque polymers are an excellent choice as contrast media since proper insertion or implantation of prostheses and implants have been achieved using radiopaque biomaterials.1–21 Common polymers are built from atoms such as carbon, hydrogen, oxygen, and nitrogen. Since these elements have low electron density and low specific gravity, these polymers are radiolucent to X-rays. Therefore, many solutions have been employed to overcome this deficiency and make the polymers radiopaque. A conventional method used for the preparation of radiopaque biomaterials is the blending of polymers with opacifying agents such as barium sulfate, zirconium dioxide, bismuth or tantalum salts. The substantial limitation of these polymeric blends is the creation of a heterogeneous environment that leads to a deterioration in the final product.22–25 Homogeneous radiopaque complexes have been synthesized by chelating heavy metal salts with appropriate polymer ligands. For instance, the synthesis of a coordination complex of uranyl nitrate and poly(methyl methacrylate) has been explored.26 However, in some cases, the leaching out of free radiopacifying agents causes the loss of physical properties and biocompatibility of polymers. Therefore, the synthesis of inherently radiopaque polymers has been considered. For this purpose, the contrast agent is covalently bonded to the polymer backbone, and thus the radiopaque polymers made via this method have permanent radiopacity.1,12,27–29 Over the years, the covalent binding of iodine atoms to monomers or preformed polymers has received great attention. Among the reactive halogens, iodine is the best choice due to its safety, low cost, and the largest mass attenuation coefficient. Iodinated moieties are the most widely used radiopacifying agents for the synthesis of radiopaque polymers due to their excellent radiopacity characteristics, high stability in non-ionic environments compared to other X-ray contrast agents, and also the non-toxicity of these radiopaque systems.3,12,30,31 In contrast to water-soluble radiopacifying agents, covalently-grafted iodine is not released in vivo from these polymers, and so this kind of polymer has medical usage.1Iodinated acrylic polymers are one of the choices that are being extensively used in pharmaceuticals as basic materials for the preparation of orthopedic cements, prostheses, and dental implants. To impart radiopacity to this kind of polymer, different methods have been investigated. In one of these techniques, instead of including additives, iodinated monomers are employed, and polymerization is carried out subsequently.32–37 For instance, iodinated acrylic monomers, such as methacrylates and methacrylamides, are copolymerized with other vinylic monomers for the preparation of radiopaque polymers.38–41 Radiopaque polymer beads have been synthesized based on the copolymerization of methyl methacrylate containing triiodobenzoate that can be used for the preparation of dentures and orthopedic bone cement.42 Also the copolymerization of MMA and 2-[2′,3′,5′-triiodobenzoyl]oxoethyl methacrylate has been investigated.43 The second route is the post-functionalization of preformed polymers with iodinated species. Poly(2-hydroxyethyl methacrylate) beads have been made radiopaque through an acylation reaction using triiodobenzoic acid, iothalamic acid, and iopanoic acid.44,45 Reactive vinyl monomers such as MMA have also been copolymerized with metal salts of vinyl monomers such as barium or zinc acrylates.26 The aim of this work is the preparation of some new biocompatible acrylic polymers based on the copolymerization of MMA and acrylic acid. The copolymer was made radiopaque via the reaction of carboxylic acid groups with 4-iodophenyl isocyanate and 3,4,5-triiodophenyl isocyanate on the polymer backbone. The thermal stability, radiopacity, and cytocompatibility behavior of the modified copolymers were particularly investigated.
2. Experimental
2.1. Material
4-Iodobenzoic acid, 4-aminobenzoic acid, sodium azide, iodine monochloride, potassium iodide, thionyl chloride, and acrylic acid were purchased from Merck (Germany), and used as obtained. MMA (Merck, Germany) was distilled before use. The initiator, 2,2′-azobis(isobutyronitrile) (AIBN), was procured from Acros Organics (New Jersey, USA), and was purified through recrystallization from methanol before use. Toluene and DMF (Merck, Germany) were dried over sodium and calcium sulfate, respectively, and kept over 4 Å molecular sieves. Acetone (analytical grade, Merck, Germany) and ethyl acetate (Merck, Germany) were used as supplied, without any further purification. 3,4,5-Triiodobenzoic acid was prepared based on the Vogel procedure.462.2. Synthesis of P(MMA-co-AA)
Into a 50 mL two-necked flask, 10 mL (0.0938 mol) of methyl methacrylate (MMA) and 1.3 mL (0.0187–20 mol%) of acrylic acid were poured into 10 mL of dried DMF. To the mixture, 0.19 g (1 mol%) of AIBN was added, and the mixture was purged in nitrogen for 30 min. The reaction was then continued at 70 °C for 2 h with constant stirring. The copolymer produced was precipitated out in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol–water mixture. The product, P(MMA-co-AA), was isolated as a white solid, filtered, and dried under reduced pressure for 48 h (7.14 g yield).
1 methanol–water mixture. The product, P(MMA-co-AA), was isolated as a white solid, filtered, and dried under reduced pressure for 48 h (7.14 g yield).
2.3. Modification of P(MMA-co-AA) with 4-iodophenyl isocyanate
2.4. Modification of P(MMA-co-AA) with 3,4,5-triiodophenyl isocyanate
3. Characterization
IR spectra were obtained on an FT-IR spectrometer (Shimadzu Model 460) with potassium bromide pellets in the range of 400–4000 cm−1. Thermal analyses were performed using a TA analyzer (Mettler Toledo, TGA 1) in a nitrogen atmosphere at a heating rate of 10 °C min−1. 1H-NMR spectra were obtained on a 300 MHz instrument (Bruker AC-300, USA) with CDCl3 used as the solvent. Elemental analysis was performed using Costech 4010 (Italy). DSC measurements were performed using a Mettler Toledo DSC 1 instrument under nitrogen atmosphere. Molecular weight distribution was determined by a GPC Agilent 1100. X-radiographs were obtained using a standard clinical X-ray machine (Xgenus, DeGotzen, Italy) at 60 keV. Energy dispersive X-ray (EDAX) analyses were conducted using a scanning electron microscope (VEGA\\TESCAN-XMU).3.1. Cytocompatibility using in vitro culture
Cytotoxicity testing of the iodinated copolymers was done via direct MTT-assay on Swiss mouse embryonic stem tissue fibroblast-like cells (NIH3T3 cell line), as reported.4. Result and discussion
4.1. Preparation and modification of copolymer
MMA is a well-known and practical monomer. This monomer can be homopolymerized or copolymerized with other acrylic monomers using known free radical initiators. The random copolymerization of MMA with ordinary acrylate monomers has been considered due to its high reactivity and numerous applications. Our study was based upon the synthesis of an inherent radiopaque copolymer via the copolymerization of MMA and acrylic acid in an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mol ratio and its modification using iodinated groups (Fig. 1). Copolymerization was carried out at 70 °C using AIBN as a free radical initiator (Fig. 2). 4-Iodophenyl isocyanate and 3,4,5-triiodophenyl isocyanate were used to impart radiopacity to the copolymer. The carboxylic acid moieties on the copolymer backbone are the sites of interest for reaction with the isocyanate functional group.
20 mol ratio and its modification using iodinated groups (Fig. 1). Copolymerization was carried out at 70 °C using AIBN as a free radical initiator (Fig. 2). 4-Iodophenyl isocyanate and 3,4,5-triiodophenyl isocyanate were used to impart radiopacity to the copolymer. The carboxylic acid moieties on the copolymer backbone are the sites of interest for reaction with the isocyanate functional group.
4.2. FT-IR spectra
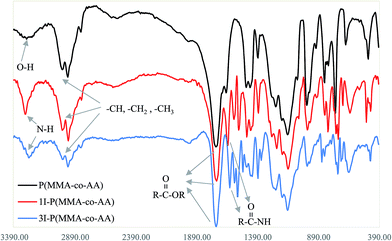
The iodinated copolymers were characterized using the FT-IR spectra, and their chemical composition was compared with the P(MMA-co-AA). As shown in Fig. 3, the peaks at 2952 and 2995 cm−1 are attributed to the stretching vibration of C–H in methyl and methylene groups. The peak at 1720 cm−1 is assigned to the stretching vibration of the ester and acid carbonyl groups. The broad peak around 3200–3600 cm−1 is assigned to the hydroxyl group of carboxylic acids. The fading of this peak and the appearance of sharp peaks at 3300 and 3370 cm−1 correspond to the N–H moiety of 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA) amide groups, respectively. The emergence of this peak confirmed the reaction of carboxylic acid and isocyanate moieties and the formation the amide functional group on the copolymer backbone. In the spectra of the iodinated copolymers, the peak at 1725 cm−1 is attributed to the stretching vibration of the ester carbonyl group. The broadening of this peak is related to the overlapping of the ester and amide carbonyl groups. The perfect disappearance of the O–H stretching peak is evidence for the complete amidation of the carboxylic acid species. The spectrum of the iodinated copolymers also showed peaks due to N–H bending and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in the region of 1450–1600 cm−1.
C stretching in the region of 1450–1600 cm−1.
4.3. Determination of copolymer composition
The amount of carboxylic acid groups on the copolymer chain can be measured by acid–base titration. For this purpose, 0.2 g of non-iodinated copolymer was dissolved in DMF and phenolphthalein was added as the indicator. The titration was carried out with a NaOH solution (0.1 M) as the titrant. The obtained results showed that 20 mol% of the sample is composed of acrylic acid. These results are also consistent with the amount of acrylic acid used for copolymerization as feedstock.4.4. Determination of iodine content
In order to further evaluate the copolymer composition and iodine content, potentiometric titration was performed for the iodinated copolymers. To this end, the 1I- and 3I-copolymers were alkali fused with sodium to release the iodine content of the samples as sodium iodide. Then titration was carried out with a standard 0.0105 M solution of AgNO3 until a drastic change in the potential occurred. The process was continued until the potential changes were halted. Investigation of the results showed 19% and 41.67% of iodine for 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA) respectively, which confirms the 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mole ratio of MMA to AA in the copolymer chain.
20 mole ratio of MMA to AA in the copolymer chain.
4.5. Elemental analysis
Elemental analysis (C, H, N, and O) was performed on the non-modified and iodinated copolymers, and the results obtained are summarized in Table 1. The experimental values for C, H, O, and N are in agreement with the theoretical amounts. Also the resulting values for the iodine content of 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA) from elemental analysis confirmed the results of the potentiometric titration, and all the results obtained were consistent with the theoretical values.| Calculated | Experimental | |||||||
|---|---|---|---|---|---|---|---|---|
| C | H | O | N | C | H | O | N | |
| P(MMA-co-AA) | 58.4 | 7.62 | 33.9 | — | 57.99 | 7.08 | 34.93 | — |
| 1I-copolymer | 51.7 | 5.94 | 21.4 | 2.08 | 51.56 | 6.34 | 18.32 | 1.9 |
| 3I-copolymer | 37.6 | 4.1 | 15.56 | 1.51 | 36.98 | 3.81 | 13.15 | 1.49 |
4.6. NMR spectroscopy
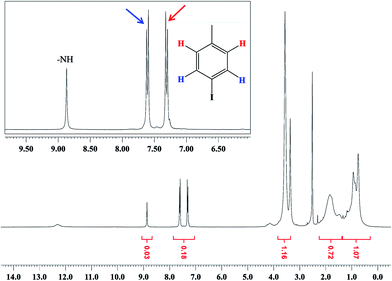
The structure of P(MMA-co-AA) was evaluated by 1H-NMR spectroscopy. The 1H-NMR spectrum confirmed the results of acid–base titration and composition of the copolymer. As shown in Fig. 4, the peak at 0.8 ppm was assigned to the methyl group of built-in MMA, and the peaks in the region of 1–2.9 ppm correspond to the methylene groups of the main chain. Also the protons of the methoxy group appear at 3.6 ppm. Accommodation of theoretical and practical calculations of the integral ratio of the methoxy group protons to the other protons proved the 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio of MMA to AA in the polymer backbone. This result is almost consistent with the ratio of the monomers as initially used.
20 ratio of MMA to AA in the polymer backbone. This result is almost consistent with the ratio of the monomers as initially used.
The 1H-NMR spectra of 1I–P(MMA-co-AA) are shown in Fig. 5. The presence of two doublets at 7–8 ppm shows the iodination of P(MMA-co-AA) through the 4-iodophenyl isocyanate molecule. Also the appearance of a singlet peak at about 8 ppm is evidence for the reaction of 3,4,5-triiodophenyl isocyanate with the copolymer (Fig. 6).
P(MMA-co-AA), 13C-NMR (300 MHz, CDCl3): δ = 23, 31, 37, 44, 52, 54 (aliphatic), δ = 176, 178 (carbonyl).
1I–P(MMA-co-AA), (300 MHz, DMSO-d6): δ = 17, 19, 44, 45, 52, 86 (aliphatic), δ = 121, 138, 140, 153 (aromatic), δ = 177, 178 (carbonyl).
3I–P(MMA-co-AA), (300 MHz, DMSO-d6): δ = 17, 19, 44, 45, 52, 54 (aliphatic), δ = 128, 129, 142, 153 (aromatic), δ = 177, 178 (carbonyl).
4.7. Gel permeation chromatography
The molecular weights (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n and
n and ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w) and molecular weight distribution were determined by GPC Agilent 1100, refractive index detector (RI – G1362A), PLgel Mixed-C, 300 × 7.5 mm, 5 μm (79911GP-MXC) columns, and isocratic pump (G1310A). Tetrahydrofuran was used as the mobile phase at a flow rate of 0.5 mL min−1. The instrument was calibrated using the polystyrene standards at 30 °C. The variations in the average molecular weight and polydispersity are summarized in Table 2. Increasing the molecular weight of 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA) related to P(MMA-co-AA) proved the iodination of P(MMA-co-AA) via mono- and triiodophenyl isocyanate, respectively. Furthermore, enhancing the weight of copolymers led to more uniform chains, and so the iodinated copolymers had a narrower molecular weight distribution and lower polydispersity values (Fig. 7).
w) and molecular weight distribution were determined by GPC Agilent 1100, refractive index detector (RI – G1362A), PLgel Mixed-C, 300 × 7.5 mm, 5 μm (79911GP-MXC) columns, and isocratic pump (G1310A). Tetrahydrofuran was used as the mobile phase at a flow rate of 0.5 mL min−1. The instrument was calibrated using the polystyrene standards at 30 °C. The variations in the average molecular weight and polydispersity are summarized in Table 2. Increasing the molecular weight of 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA) related to P(MMA-co-AA) proved the iodination of P(MMA-co-AA) via mono- and triiodophenyl isocyanate, respectively. Furthermore, enhancing the weight of copolymers led to more uniform chains, and so the iodinated copolymers had a narrower molecular weight distribution and lower polydispersity values (Fig. 7).
| Sample code | ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n n |
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w w |
Polydispersity |
|---|---|---|---|
| P(MMA-co-AA) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 334 334 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 717 717 |
4.327 |
| 1I–P(MMA-co-AA) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 809 809 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255 255 |
2.8475 |
| 3I–P(MMA-co-AA) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 313 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 807 807 |
2.6981 |
4.8. Energy dispersive X-ray analysis
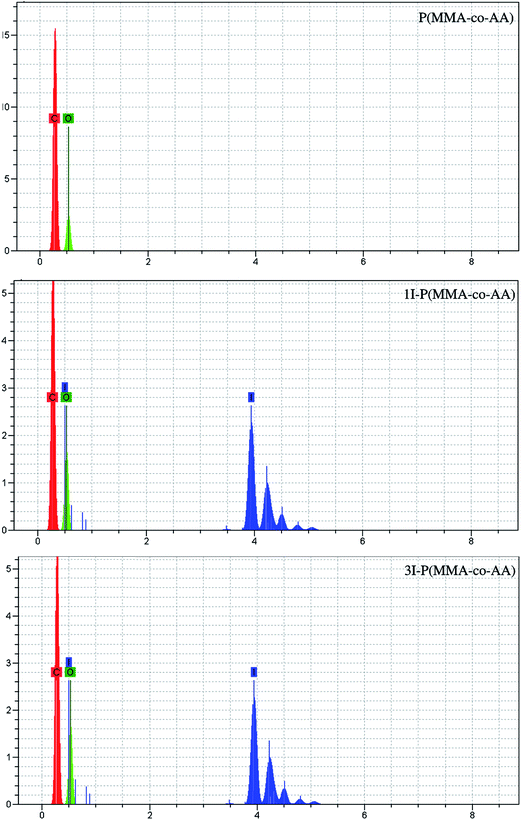
The energy dispersive X-ray spectra of the non-iodinated and iodinated copolymers are shown in Fig. 8. The EDAX spectra for the non-iodinated copolymer show just the peaks for carbon and oxygen elements, whereas the presence of iodine atoms in the structure of the iodinated copolymer is well-established. The peaks at about 3.5–5 keV in the EDAX spectra can be attributed to the existence of iodine atoms in the copolymer chain.4.9. Thermal analysis
Thermal properties of the copolymers were evaluated by TGA. The thermograms show that the 1I-copolymer has a lower thermal stability than the non-modified copolymer, whereas the 3I-copolymer is stable to higher temperatures, and shows greater thermal stability than the non-iodinated copolymer. As shown in Fig. 9, the non-modified copolymer had a 5% weight loss at 218 °C, whereas in the case of iodinated copolymers, the T5 values for 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA) were 160 and 246 °C respectively. In fact, introducing the iodine moiety onto the copolymer backbone enhances the steric hindrance and, therefore, reduces the inter-chain van-der-Waals interactions that decrease the thermal stability of 1I–P(MMA-co-AA) compared with the non-iodinated sample. On the other hand, increasing the number of iodine atoms from 1 to 3 in the repeating unit of the copolymer increases the rigidity and inflexibility of the copolymer, and so the 3I–P(MMA-co-AA) sample has the highest initiation degradation temperature. The weight loss (about 30.16%) for 1I–P(MMA-co-AA) occurred at 280 °C, which is attributed to the separation of the 4-iodophenyl amine groups from the polymer backbone. The DTG curve for the 3I–P(MMA-co-AA) sample shows a sharp peak (50% weight loss) at 320 °C, which is related to the separation of the 3,4,5-triiodophenyl amine group as a result of amide bond cleavage.The DSC traces of P(MMA-co-AA), 1I–P(MMA-co-AA), and 3I–P(MMA-co-AA) are shown in Fig. 10. The Tg temperature for the non-modified copolymer was about 82 °C, whereas those for the 1I- and 3I-copolymers were about 76 and 73 °C, respectively. The decrease in the Tg values in the iodinated copolymers can be attributed to the steric hindrance of the hanging iodinated groups, which caused an increase in the free space between the chains. Also as a result of the iodination of P(MMA-co-AA) using 4-iodophenyl isocyanate and 3,4,5-triiodophenyl isocyanate, the carboxylic acid groups on the main chain were replaced by the amide groups that decreased the intra-molecular interactions and the Tg temperature.
4.10. Radiopacity
X-radiography was carried out on the non-iodinated and iodinated copolymers, which were compared with an aluminium wedge from 0.5 to 3 mm (Fig. 11). All the samples were prepared as 2 mm thick disks. X-radiography showed excellent radiopacity for 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA), whereas non-modified samples, due to their lack of heavy elements, have no significant X-ray visibility. Among the samples, the 3I-copolymer has the highest opacity, and is about equal to a 2 mm aluminium wedge. | ||
| Fig. 11 X-ray image of aluminium wedge, non-iodinated copolymer (A), 1I–P(MMA-co-AA) (B), and 3I–P(MMA-co-AA) (C). | ||
4.11. Cytocompatibility
In order to evaluate the toxicity of the iodinated copolymers, and also their biocompatibility, a direct MTT assay was carried out on Swiss mouse embryonic stem tissue fibroblast-like cells (NIH3T3 cell line) according to the ISO10993-5 standard. For this purpose, the fibroblast 3T3 cell line was incubated for 24 h in a culture medium containing 10% fetal bovine serum and 1% streptomycin at 37 °C. The iodinated copolymers were sterilized in an autoclave, and were directly exposed to the cells (Fig. 12). After 24 h incubation, the cells were washed with PBS buffer, and subsequently, 10 μL of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye in 5 mg mL−1 was added. The incubation was carried out for 4 h, and the dye was extracted using DMSO. The optical absorption of MTT was measured at a wavelength of 550 nm. Evaluation of the results for 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA) showed that direct contact of the copolymers with the cells had no adverse consequence on cell viability, and the iodinated copolymers were considered to be non-toxic. | ||
| Fig. 12 Fibroblast 3T3 cells (a and b), various amounts of 1I-copolymer (c–e), and 3I-copolymer (f–h) around the fibroblast cells. | ||
5. Conclusion
The P(MMA-co-AA) samples were synthesized based on copolymerization of MMA and acrylic acid. The copolymer composition was determined via 1H-NMR spectroscopy, acid–base and potentiometric titration. 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA) were subsequently prepared through the reaction of P(MMA-co-AA) with 4-iodophenyl isocyanate and 3,4,5-triiodophenyl isocyanate groups, respectively. Covalent attachment of iodinated moieties to the main backbone imparted radiopacity to the copolymer. Increasing the number of iodine atoms in the copolymer structure enhanced radiopacity, which is comparable with that of an aluminium wedge. Elemental analysis, EDAX spectra, FT-IR, and NMR spectroscopy confirmed the structures of the non-modified and iodinated copolymers. Also the molecular weight distribution and polydispersity of the samples were evaluated. Increasing the weight of 1I–P(MMA-co-AA) and 3I–P(MMA-co-AA) relative to the non-modified copolymer led to a uniform molecular weight distribution, and is also evidence for the iodination of the P(MMA-co-AA). Biocompatibility and non-cytotoxicity of the iodinated copolymers were proved via direct MTT-assay. The obtained images showed that exposure of iodinated copolymers to Swiss mouse embryonic stem tissue fibroblast-like cells (NIH3T3 cell line) had no corrupting influence on cell proliferation.Acknowledgements
The financial support of Shahrood University of Technology is gratefully acknowledged. We are also grateful to Dr Salman Gharagozloo and Dr Hossein Rahimi for their help in measuring the radiopacity of the samples. The authors also wish to acknowledge Dr Behrooz Yahyaei for his assistance with the MTT-assay.References
- S. Kiran and R. Joseph, J. Biomed. Mater. Res., Part A, 2015, 103, 2214–2224 CrossRef CAS PubMed.
- J.-M. Yang, Biomaterials, 1997, 18, 1293–1298 CrossRef CAS PubMed.
- X. Wang, X. Geng, L. Ye, A.-Y. Zhang and Z.-G. Feng, Frontiers of Materials Science, 2010, 4, 366–375 CrossRef.
- R. R. Vivan, R. Ordinola-Zapata, C. M. Bramante, N. Bernardineli, R. B. Garcia, M. A. H. Duarte and I. G. de Moraes, Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 2009, 108, e35–e38 CrossRef PubMed.
- L. Sang, Z. Wei, K. Liu, X. Wang, K. Song, H. Wang and M. Qi, J. Biomed. Mater. Res., Part A, 2014, 102, 1121–1130 CrossRef PubMed.
- G. Pekkan, A. Aktas and K. Pekkan, Journal of Cranio-Maxillofacial Surgery, 2012, 40, e1–e4 CrossRef PubMed.
- F. Mottu, D. A. Rüfenacht, A. Laurent and E. Doelker, Biomaterials, 2002, 23, 121–131 CrossRef CAS PubMed.
- D. Mawad, H. Mouaziz, A. Penciu, H. Mehier, B. Fenet, H. Fessi and Y. Chevalier, Biomaterials, 2009, 30, 5667–5674 CrossRef CAS PubMed.
- S. Kiran, N. R. James, A. Jayakrishnan and R. Joseph, J. Biomed. Mater. Res., Part A, 2012, 100, 3472–3479 CrossRef CAS PubMed.
- N. R. James and A. Jayakrishnan, Biomaterials, 2007, 28, 3182–3187 CrossRef CAS PubMed.
- A. Hagit, B. Soenke, B. Johannes and M. Shlomo, Biomacromolecules, 2010, 11, 1600–1607 CrossRef CAS PubMed.
- P. Ghosh, M. Das, A. P. Rameshbabu, D. Das, S. Datta, S. Pal, A. B. Panda and S. Dhara, ACS Appl. Mater. Interfaces, 2014, 6, 17926–17936 CAS.
- A. Galperin and S. Margel, Biomacromolecules, 2006, 7, 2650–2660 CrossRef CAS PubMed.
- S. Dawlee, A. Jayakrishnan and M. Jayabalan, J. Mater. Sci.: Mater. Med., 2009, 20, 243–250 CrossRef PubMed.
- S. Dawlee and M. Jayabalan, J. Biomater. Appl., 2012, 28, 28–37 CrossRef PubMed.
- K. Davy, M. Anseau and C. Berry, J. Dent., 1997, 25, 499–505 CrossRef CAS PubMed.
- A. L. Carbone, M. Song and K. E. Uhrich, Biomacromolecules, 2008, 9, 1604–1612 CrossRef CAS PubMed.
- E. J. Boelen, C. S. van Hooy-Corstjens, S. K. Bulstra, A. Van Ooij, L. W. van Rhijn and L. H. Koole, Biomaterials, 2005, 26, 6674–6683 CrossRef CAS PubMed.
- G. Agusti, O. Jordan, G. Andersen, É. Doelker and Y. Chevalier, J. Appl. Polym. Sci., 2015, 132, 41791–41803 CrossRef.
- K. A. Aamer, K. L. Genson, J. Kohn and M. L. Becker, Biomacromolecules, 2009, 10, 2418–2426 CrossRef CAS PubMed.
- N. Moszner, U. Salz, A. M. Klester and V. Rheinberger, Angew. Makromol. Chem., 1995, 224, 115–123 CrossRef CAS.
- H. R. Rawls, R. J. Granier, J. Smid and I. Cabasso, J. Appl. Biomater., 1996, 31, 339–343 CAS.
- J. Pariente, L. Bordenave, R. Bareille, C. Ohayon-Courtes, C. Baquey and M. Le Guillou, Biomaterials, 1999, 20, 523–527 CrossRef CAS PubMed.
- M. Ginebra, L. Albuixech, E. Fernandez-Barragan, C. Aparicio, F. Gil, J. San Roman, B. Vazquez and J. Planell, Biomaterials, 2002, 23, 1873–1882 CrossRef CAS PubMed.
- S. Deb, S. Abdulghani and J. Behiri, Biomaterials, 2002, 23, 3387–3393 CrossRef CAS PubMed.
- I. Cabasso, J. Smid and S. K. Sahni, J. Appl. Polym. Sci., 1989, 38, 1653–1666 CrossRef CAS.
- N. R. James, J. Philip and A. Jayakrishnan, Biomaterials, 2006, 27, 160–166 CrossRef CAS PubMed.
- S. Kiran, N. R. James, R. Joseph and A. Jayakrishnan, Biomaterials, 2009, 30, 5552–5559 CrossRef CAS PubMed.
- Q. Yin, F. Y. Yap, L. Yin, L. Ma, Q. Zhou, L. W. Dobrucki, T. M. Fan, R. C. Gaba and J. Cheng, J. Am. Chem. Soc., 2013, 135, 13620–13623 CrossRef CAS PubMed.
- H. Lusic and M. W. Grinstaff, Chem. Rev., 2012, 113, 1641–1666 CrossRef PubMed.
- A. Boyde, F. A. Mccorkell, G. K. Taylor, R. J. Bomphrey and M. Doube, Microsc. Res. Tech., 2014, 77, 1044–1051 CrossRef CAS PubMed.
- C. S. van Hooy-Corstjens, K. Saralidze, M. L. Knetsch, P. J. Emans, M. W. de Haan, P. C. Magusin, B. Mezari and L. H. Koole, Biomacromolecules, 2007, 9, 84–90 CrossRef PubMed.
- M.-A. B. Kruft, A. Benzina, R. Blezer and L. H. Koole, Biomaterials, 1996, 17, 1803–1812 CrossRef CAS PubMed.
- A. Benzina, M. A. B. Kruft, R. Blezer, T. Lindhout, L. H. Koole, F. H. van der Veen and F. H. Bär, J. Appl. Biomater., 1996, 32, 459–466 CAS.
- M. Kruft, A. Benzina, F. Bär, F. Van der Veen, C. Bastiaansen, R. Blezer, T. Lindhout and L. Koole, J. Appl. Biomater., 1994, 28, 1259–1266 CAS.
- C. S. van Hooy-Corstjens, L. E. Govaert, A. B. Spoelstra, S. K. Bulstra, G. M. Wetzels and L. H. Koole, Biomaterials, 2004, 25, 2657–2667 CrossRef CAS PubMed.
- A. Artola, M. Gurruchaga, B. Vazquez, J. San Roman and I. Goni, Biomaterials, 2003, 24, 4071–4080 CrossRef CAS PubMed.
- S. Lakshmi, N. R. James, V. Nisha and A. Jayakrishnan, J. Appl. Polym. Sci., 2003, 88, 2580–2584 CrossRef CAS.
- B. Vazquez, M. Ginebra, F. Gil, J. Planell, A. L. Bravo and J. San Román, Biomaterials, 1999, 20, 2047–2053 CrossRef CAS PubMed.
- M. Okamura, T. Yamanobe, T. Arai, H. Uehara, T. Komoto, S. Hosoi and T. Kumazaki, J. Mol. Struct., 2002, 602, 17–28 CrossRef.
- M. Okamura, H. Uehara, T. Yamanobe, T. Komoto, S. Hosoi and T. Kumazaki, J. Mol. Struct., 2000, 554, 35–45 CrossRef CAS.
- K. Davy, M. Anseau, M. Odlyha and G. Foster, Polym. Int., 1997, 43, 143–154 CrossRef CAS.
- C. Zaharia, T. Zecheru, M. F. Moreau, F. Pascaretti-Grizon, G. Mabilleau, B. Marculescu, R. Filmon, C. Cincu, G. Staikos and D. Chappard, Acta Biomater., 2008, 4, 1762–1769 CrossRef CAS PubMed.
- A. Jayakrishnan, B. C. Thanoo, K. Rathinam and M. Mohanty, J. Appl. Biomater., 1990, 24, 993–1004 CAS.
- D. Horak, M. Metalova, F. Švec, J. Drobnik, J. Kalal, M. Borovička, A. Adamyan, O. Voronkova and K. Gumargalieva, Biomaterials, 1987, 8, 142–145 CrossRef CAS PubMed.
- A. Hannaford, B. Furniss, V. Rogers, P. Smith and A. Tatchell, Vogel's Textbook of Practical Organic Chemistry, Including Qualitative Organic Analysis, 5th edn, Longman, 1978 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |