Highly porous three-dimensional carbon nanotube foam as a freestanding anode for a lithium-ion battery
Abstract
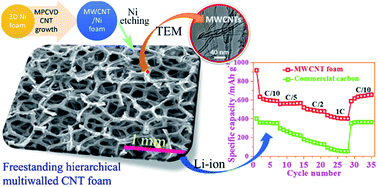
Anodes composed of freestanding, binder-free and hierarchical multiwalled carbon nanotube (MWCNT) foam have been demonstrated. These three-dimensional MWCNT foams are fabricated using a Ti–Al–Fe trilayer catalyst on Ni-foam through a microwave plasma assisted chemical vapor deposition. The MWCNT foam possesses a hierarchical graphitic microstructure, high porosity (99.8%), reduced impedance and specific capacitance of 790 mA h g−1 when cycled between 0 and 3 V for a lower current density (0.1C). At a higher current density (1C), the foam electrode retains a discharge capacity of 390 mA h g−1, significantly higher than that of the commercial graphite anode. Upon extended charge–discharge cycling, MWCNT foams shows stable capacities of 790 and 510 mA h g−1 at current densities of 0.1C and 1C respectively, maintaining a high coulombic efficiency of 99.7%. Preserved structural and chemical stability of the MWCNT foams during lithiation–delithiation cycling can be utilized as a basis for improved electrochemical energy storage in CNT based architectures.

 Please wait while we load your content...
Please wait while we load your content...