Uniform polymer beads by membrane emulsification-assisted suspension polymerisation
Abstract
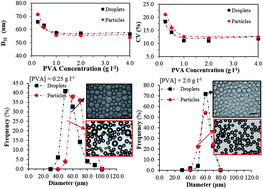
This work focuses on a two-stage polymerisation process for the production of uniform polymer beads. Highly uniform droplets were firstly produced by a stirred-vessel membrane emulsification device. Methyl methacrylate (MMA) and a specific grade of polyvinyl alcohol (PVA) were used as monomer and stabiliser, respectively. The effects of various process parameters affecting the droplet size and uniformity including feeding policy, agitation speed, stabiliser concentration, and flowrate were investigated. The evolution of droplet size and its coefficient of variation (CV) were monitored over the course of emulsification. A new start-up policy, validated by monitoring droplet formation at the membrane surface, was introduced that eliminated the non-uniformity in the size of droplets formed early during emulsification. The mechanisms contributing to droplet size distribution broadening at the membrane surface during formation were decoupled from those acting in the emulsification vessel during circulation. The high CV obtained at low PVA concentration and high agitation speed was attributed to drop breakup and coalescence occurring in the emulsification vessel, respectively, after droplets formed. The emulsification was followed by a shear-controlled suspension polymerisation to convert the discrete droplets of monomer to polymer beads. A wide range of reactor impeller speeds and PVA concentrations was studied to find the conditions under which the droplets formed via membrane emulsification would not undergo further break-up or coalesce during polymerisations and a one-to-one copy of the initial droplets with the same CV can be achieved.

 Please wait while we load your content...
Please wait while we load your content...