Carbon nanotube-induced migration of silver nanowire networks into plastic substrates via Joule heating for high stability†
Jong Seok Wooab,
Byung Kuk Kima,
Ho Young Kima,
Geon-Woong Leea,
Soo-Young Park*b and
Joong Tark Han*a
aNano Hybrid Technology Research Center, Korea Electrotechnology Research Institute, Changwon, 51543, Republic of Korea. E-mail: jthan@keri.re.kr
bMajor in Polymer Science and Engineering, School of Applied Chemical Engineering, Kyungpook National University, #1370 Sangyuk-dong, Buk-gu, Daegu, 41566, Republic of Korea. E-mail: psy@knu.ac.kr
First published on 5th September 2016
Abstract
The hydrothermal and mechanical stability of transparent conducting films is a prerequisite for commercial applications in optoelectronic devices. However, the environmental stability of silver nanowire (AgNW) transparent conducting films (TCFs) is not promising without post-treatment or overcoating with foreign materials. Herein, we reported that thermal embedding of silver nanowire networks assisted by single-walled carbon nanotubes (SWCNTs) via Joule heating is a straightforward method to enhance the hydrothermal stability and interfacial adhesion of flexible AgNW TCFs on plastic substrates. Under a direct current flow, the effective Joule heating of SWCNTs, which are thermally stable and compatible with hydrophobic substrates, led to the migration of hydrophilic AgNWs into the hydrophobic polycarbonate (PC) substrate without deforming it. The resulting flexible and transparent AgNW-embedded PC film exhibited long-term stability at high temperatures during heating and cooling cycles under high applied voltages, without requiring further passivation with other materials; therefore, it has the potential to be used in a broad range of applications such as optoelectronic devices and flexible thin-film heaters.
Introduction
Flexible transparent conducting films (TCFs) based on one-dimensional (1D) and two-dimensional (2D) conducting nanomaterials such as metal nanowires, single-walled carbon nanotubes (SWCNTs), graphene oxide, and graphene have been extensively studied as inexpensive and efficient alternatives to TCFs based on indium-tin oxide (ITO) in optoelectronic applications.1–8 Among the low-dimensional conducting nanomaterials, metal nanowires with random networks are promising candidates for optoelectronic devices because of their excellent electrical conductivity, optical transparency, and flexibility. In particular, silver nanowires (AgNWs) have received much attention for real-life industrial applications.9–13 However, AgNWs have poor electrical stability, which results in their rupture at junctions under high applied current; moreover, they are hampered by poor oxidation resistance and thermal stability (even at 200 °C), which lead to an increase in the sheet resistance (Rs). A new strategy to overcome these problems is to use hybrid structures of AgNWs with 1D and 2D nanomaterials such as carbon nanotubes, graphene oxide, and graphene.14–22 Moreover, Jiang et al. demonstrated improvement of the surface roughness and mechanical robustness of AgNWs by burying them below a polymer matrix.23 Huang et al. fabricated a AgNW-embedded PI film for a flexible heater.24 Li et al. developed a novel heat-resistant coating over the AgNWs on a polyacrylate substrate.25 However, these transparent AgNWs did not fully satisfy the requirements for commercialization because of the transfer and over-coating methods used for passivation. To realize high performance of TCFs with 1D conducting nanomaterials, the interfacial structure between the substrate and the conducting material or between conducting materials must be systematically optimized. Moreover, for practical application of TCFs, optimization of the extrinsic properties (e.g. environmental stability, interfacial adhesive properties, and surface hardness) and intrinsic optoelectrical properties (e.g. Rs vs. transmittance (T)) is a prerequisite. In this context, chemical or thermal welding is one of the most efficient methods to passivate 1D conducting nanomaterials on plastic substrates without requiring a another coating over the nanowires.26,27 However, the mismatch between the surface tension of the metal nanowires and the hydrophobic plastic substrate limits self-migration of the nanowires into the plastic substrate, and leads to environmental instability of the metal-nanowire networks.In this paper, we demonstrated that the SWCNTs deposited on AgNWs promoted the migration of the latter into the plastic substrate under a current flow by Joule heating without distorting the substrate. This is attributed to favourable interactions between the SWCNTs and the hydrophobic plastic substrate as well as the stable Joule heating of SWCNT networks on AgNW films. This SWCNT-assisted self-passivation of AgNW networks is a promising method to fabricate hydrothermally stable and flexible TCFs.
Results and discussion
Flexible TCFs coated with AgNWs or SWCNTs (AgNW or SWCNT films) were prepared by directly air-spraying an aqueous dispersion of AgNWs or SWCNTs, stabilized by sodium dodecylbenzenesulfonate, on polycarbonate (PC) substrates. The welding behaviour of a AgNW film on the PC substrate was first investigated by thermal treatment in a convection oven at 150 °C for 3 h. Fig. 1a shows an AFM image of the AgNW film after the thermal treatment. The AgNWs were not fully embedded in the PC substrate. The height profile of the AFM image in Fig. 1b clearly shows partial welding of the AgNWs. The AgNWs underneath the junctions were embedded deeper within the substrate than the AgNWs at other locations because of the weight of the upper AgNWs at the junctions, as illustrated in Fig. 1c. The surface-tension mismatch between the AgNWs (∼500 mN m−1 of liquid silver in air) and the hydrophobic PC substrate (∼34.2 mN m−1) prevented the AgNWs from completely embedding in the plastic substrate, as illustrated in Fig. 1d.The wettability of a solid surface can be determined by a Zisman plot, which measures variations in the cosine of the contact angle θ as a function of the surface tension γ for different liquids. The critical surface tension γc, extrapolated from the plot at cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 1, is a characteristic of the solid and is independent of the wetting substances.28 For a given surface, γmax is the cutoff value of surface tension when cos
θ = 1, is a characteristic of the solid and is independent of the wetting substances.28 For a given surface, γmax is the cutoff value of surface tension when cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0. Substances with γ < γc undergo complete wetting, and those with γc < γ < γmax undergo partial wetting upon formation of a thin film. When γ > γmax, the liquid does not cause wetting. The surface tension on a polymer substrate lies in the range 30–50 mN m−1, which is much lower than that for silver in air. Therefore, the molten PC chains did not wet the AgNWs because of the large difference between the values of their surface tension. The force generated by the difference in the surface tension may have even caused the AgNWs to float at high temperatures. Another method to anneal the AgNWs is to apply an electric current to the conducting AgNWs. In this study, the AgNW films (Rs ≈ 50 Ω sq.−1; T > 90%) were used to fabricate TCF heaters. Fig. 2a shows the temperature change of the AgNW films as a function of time at applied input voltages of 5 and 10 V. Unfortunately, the AgNW films showed the presence of hot spots and a temperature drop even at a low heating temperature of ∼75 °C and at a low input voltage of 10 V. The FESEM image in Fig. 2b clearly shows the AgNWs breaking apart at the junction, which was due to the generation of hot spots resulting from the high junction electrical resistance, as illustrated in Fig. 2c. Moreover, the limited electrical heating did not allow the migration of AgNWs into the PC substrate without the application of external forces.
θ = 0. Substances with γ < γc undergo complete wetting, and those with γc < γ < γmax undergo partial wetting upon formation of a thin film. When γ > γmax, the liquid does not cause wetting. The surface tension on a polymer substrate lies in the range 30–50 mN m−1, which is much lower than that for silver in air. Therefore, the molten PC chains did not wet the AgNWs because of the large difference between the values of their surface tension. The force generated by the difference in the surface tension may have even caused the AgNWs to float at high temperatures. Another method to anneal the AgNWs is to apply an electric current to the conducting AgNWs. In this study, the AgNW films (Rs ≈ 50 Ω sq.−1; T > 90%) were used to fabricate TCF heaters. Fig. 2a shows the temperature change of the AgNW films as a function of time at applied input voltages of 5 and 10 V. Unfortunately, the AgNW films showed the presence of hot spots and a temperature drop even at a low heating temperature of ∼75 °C and at a low input voltage of 10 V. The FESEM image in Fig. 2b clearly shows the AgNWs breaking apart at the junction, which was due to the generation of hot spots resulting from the high junction electrical resistance, as illustrated in Fig. 2c. Moreover, the limited electrical heating did not allow the migration of AgNWs into the PC substrate without the application of external forces.
Based on these results, we hypothesized that the limited self-migration of AgNWs into the PC substrate can be overcome by combining the AgNWs with a conducting and electrically stable 1D material whose surface tension is similar to that of the hydrophobic PC substrate. Dujardin et al. reported that the surface tension of CNTs falls within the range of 40–80 mN m−1, which is similar to the values for hydrophobic polymeric materials.29,30 Therefore, highly conducting SWCNTs are good candidates for triggering the migration of AgNWs into the plastic substrate during heating. We first investigated the thermal migration behaviour of SWCNT films without a AgNW coating. The SWCNT films had Rs in the range of 100–1000 Ω sq.−1, and transmittance (T) in the range of 78–95%. Fig. 3a shows the temperature of the SWCNT films (100 Ω sq.−1) as a function of time under an applied voltage of 30 V; the temperature reached a saturated (steady-state) value of 120 °C. The infrared thermal image of a SWCNT film (inset of Fig. 3a) shows that the temperature on the entire film was uniform under the applied voltage. This even distribution of the generated heat may be attributed to the excellent thermal and electrical conductivity of the SWCNTs as well as the high thermal stability of the SWCNTs, which was maintained even at high temperatures. The surface temperature of a SWCNT film depended on the supplied DC voltage and the Rs value of the film. Fig. 3b shows the temperature generated within a SWCNT film as a function of the applied voltage at different Rs values. The temperature increased almost linearly with an increase in the applied voltage for all values of Rs under study, and the slope decreased as Rs increased. A steep rise in the temperature of the SWCNT film was observed at low Rs (e.g. 100 Ω sq.−1). However, the high temperature (>160 °C) led to distortion and melting of the PC substrate (see inset of Fig. S1c†).
Fig. 3c–e show FESEM images of the SWCNT films at different temperatures. The temperature was controlled by Rs and the applied voltage. Embedding of SWCNTs by self-migration into the PC substrates was not observed at 50 °C (Fig. 3c), partial embedding was observed at 120 °C (Fig. 3d), and complete embedding occurred at 150 °C (Fig. 3e). This thermal embedding of the SWCNT film, schematically shown in Fig. 3f, affected the geometry of the SWCNTs. For example, the shift of the G+ band in the Raman spectrum of the SWCNT film (Fig. S2a†) shows the deformation of the SWCNTs by self-migration into the PC substrate. The obtained SWCNT film exhibited high hydrothermal stability at 80% relative humidity and at a temperature of 80 °C without requiring an overlying coating (Fig. S2b†). The operational stability of the SWCNT film was examined during heating and cooling cycles by alternately turning the DC power on and off, as shown in Fig. S2c.† The current flow ranged from 0.28 A (with the DC power on) to 0.30 A (with the DC power off) during the heating and cooling cycles at 30 V, which clearly indicate the SWCNTs were thermally embedded in the PC substrate. At 20 V, however, no significant change in the current was measured in the SWCNT film because of insufficient Joule heating for the thermal embedding of SWCNTs. Thus, we found that the SWCNT film (Rs = 100 Ω sq.−1) remained stable during the heating and cooling cycles at an applied voltage of 30 V.
In the context of efficient heating and embedding of 1D conducting nanomaterials, complete embedding of the SWCNT film in the PC substrate was limited by the high network density and Rs of the SWCNTs (Fig. 3). In order to overcome these problems, we studied PC substrates that were sequentially coated with AgNWs and SWCNTs to form a AgNW–SWCNT film. First, the power consumption of the SWCNT and AgNW–SWCNT films was calculated by Joule's first law, P = V2/R (where P is the power dissipated in a resistive conductor, V is the applied voltage, and R is the total resistance), as shown in Table S1.† High DC voltages must be applied to the SWCNT film to achieve complete embedding of the SWCNTs in the PC substrate because of the high Rs value of SWCNTs (Rs = 100 Ω sq.−1). In the case of the AgNW–SWCNT film, the combination of AgNWs and SWCNTs resulted in a synergistic effect for stable and efficient Joule heating because the AgNW–SWCNT film caused high power consumption owing to its low Rs (caused by the AgNWs) and surface-tension compatibility (caused by the SWCNTs). Therefore, we attempted to more effectively embed AgNWs in the PC substrate by sequentially depositing AgNWs and SWCNTs on the PC substrate, as shown in Fig. 4a. The sequential deposition was realized by over-coating SWCNTs on a AgNW film (Rs = 40 Ω sq.−1) on the PC substrate. The Rs value of this hybrid material was compared to that in an equivalent circuit in which two singular components of AgNWs and SWCNTs are connected as resistors in parallel. Fig. S3† shows the calculated and measured Rs (Rcal and Rexp, respectively) values as functions of Rs of the AgNW film when Rs of the SWCNT film was fixed at 250 Ω sq.−1. Rexp is lower than Rcal because of the low network density of the AgNW–SWCNT film owing to the reduced junction resistance of the SWCNT-connected AgNW networks.
The temperature profiles of the AgNW–SWCNT thin-film heater in Fig. 4b and c clearly show that the AgNWs were electrically stable even at 20 V, in stark contrast to the behaviour of the AgNW thin-film heater (Fig. 2). Fig. 4d exhibits the temperature profiles of the AgNW–SWCNT film during heating and cooling cycles at applied voltages of 10 and 15 V. The temperature profiles were almost the same for the first 10 cycles, indicating that the AgNW–SWCNT film was quite stable and the SWCNTs stabilized the AgNWs electrically on the PC substrate. Fig. 5 shows FESEM and AFM images of the surface of the AgNW–SWCNT film after a voltage of 20 V was applied. These images clearly demonstrate that the AgNWs were fully embedded in the plastic substrate after electrical heating, in stark contrast to AgNWs in the AgNW film shown in Fig. 1a. The AFM height profiles (Fig. 4e) also show that the AgNW–SWCNT film was embedded in the substrate after heating (see red and blue triangles). Thus, the SWCNTs played an important role in stable heating under a current flow and efficient embedding of AgNWs in the PC substrate because the surface tension of the SWCNTs was compatible with that of the substrate.
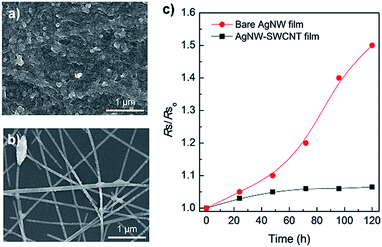
This self-passivation by thermal embedding improved the mechanical and hydrothermal stability of the film. The interfacial adhesion of AgNWs and SWCNTs in the AgNW–SWCNT film was estimated by a peel-off test. Table S2† shows the results of the peel-off test for the SWCNT and AgNW–SWCNT films at different Rs values under different applied voltages. Before thermal embedding, the AgNW and AgNW–SWCNT films were easily peeled off. However, the fully embedded SWCNT (100 Ω sq.−1 at 40 V; 100 Ω sq.−1 at 50 V) and AgNW–SWCNT films (100 Ω sq.−1 at 40 V; 40 Ω sq.−1 at 20 V; 20 Ω sq.−1 at 15 V) remained well adhered to the PC substrates. Fig. S4† indicates that Rs of the AgNW–SWCNT film did not change after the peeling test. Moreover, the transmittance of the film after Joule heating just decreased slightly because of the morphological change as shown in Fig. S5.† Fig. 6 shows FESEM images of the AgNW–SWCNT and AgNW films after the hydrothermal test at 80 °C and 80% relative humidity in air. The morphology of the AgNW–SWCNT film remained almost unchanged after exposure to these conditions (Fig. 6a), while the bare AgNW film was severely oxidized (Fig. 6b), indicating that the embedding of AgNWs in the PC substrate dramatically enhanced the hydrothermal stability of the film. This environmental stability also improved the electrical properties of the film. Fig. 6c shows the change in relative Rs of the AgNW and AgNW–SWCNT films as a function of time. The Rs value of the AgNW–SWCNT film remained stable for 5 days, while the AgNW film showed a steep increase in Rs after 50 h. This clearly indicates the advantages of the AgNW–SWCNT film.
Conclusions
We have demonstrated that SWCNTs can assist the complete embedding of AgNWs in the PC substrate via Joule heating under a direct current flow. Complete embedding of the AgNWs in the substrate was achieved by effective electrical heating of AgNW–SWCNT hybrid networks and by ensuring surface-tension compatibility between the SWCNTs and the hydrophobic PC substrate. To the best of our knowledge, this is a novel approach to fabricate fully embedded AgNWs on a PC substrate via the self-migration of AgNWs. The hybrid film also showed high environmental stability against humidity without the need for further over-coating. Furthermore, the flexible AgNW–SWCNT film showed high performance in heating and cooling operational stability and good adhesion to the substrate.Experimental
Preparation of SWCNT and AgNW solutions and film fabrication
The SWCNTs produced by arc discharge and purified by thermal treatment (Extube, Nano Solutions, Korea) were dispersed by bath sonication for 1 h and horn sonication for 1 h in a 1 wt% sodium dodecylbenzenesulfonate solution with a concentration of 1 g L−1. The mixture was then purified by two rounds of centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 60 min to remove impurities such as amorphous carbon and residual catalyst particles. The supernatant solution was deposited on PC substrates using a spray coater (NCS-400, NCS Co. Ltd., Korea). The surfactant was removed by dipping the coated substrate in deionized water twice for 10 min, followed by air drying at 80 °C for 5 min. Aqueous AgNWs with an average length of 25 μm and an average diameter of 35 nm (0.5 wt%), containing 0.07 wt% polyvinylpyrrolidone, were purchased from Nanopyxis Inc., Korea, and used as received.
000 rpm for 60 min to remove impurities such as amorphous carbon and residual catalyst particles. The supernatant solution was deposited on PC substrates using a spray coater (NCS-400, NCS Co. Ltd., Korea). The surfactant was removed by dipping the coated substrate in deionized water twice for 10 min, followed by air drying at 80 °C for 5 min. Aqueous AgNWs with an average length of 25 μm and an average diameter of 35 nm (0.5 wt%), containing 0.07 wt% polyvinylpyrrolidone, were purchased from Nanopyxis Inc., Korea, and used as received.
Fabrication of SWCNT and AgNW–SWCNT thin-film heaters
The transparent thin-film heater was fabricated with a two-terminal side-contact configuration. A DC voltage was supplied by a power supply to the heater through the screen-printed silver paste and copper contact at the film's edge. The copper layer was used to enhance the contact between the silver paste and the AgNW–SWCNT networks. AgNW–SWCNT films with an area of 4 × 7 cm2 were fabricated.Characterization of TCFs
The sheet resistance of the film was measured using a four-probe tester (Loresta MCP-T610, Mitsubishi Chemical Analytech Co., Japan). The transmittance of each film was measured using an ultraviolet-visible-near-infrared (UV-vis-NIR) spectrometer (Cary 5000, Varian, now Agilent Technologies, USA). The corresponding images of the resulting films were obtained by a field-emission scanning electron microscope (FESEM; S4800, Hitachi Corp., Japan) and an atomic force microscope (AFM; DI Nanoscope Dimension 3100, Bruker, Germany (formerly Veeco Instruments, USA, which acquired Digital Instruments, USA)). Raman spectra were measured to characterize the electronic structure of the SWCNTs at 25 °C, using a high-resolution Raman spectrometer (NTEGRA Spectra, NT-MDT, Russia) with an excitation wavelength of 633 nm. The temperature profile of each film was measured using an IR thermal imager (TH9100, NEC San-ei Instruments, Ltd., Japan) and a thermocouple (CENTER 300, Center Technology Corp., Taiwan).Acknowledgements
This work was supported by the Center for Advanced Soft-Electronics funded by the Ministry of Science, ICT and Future Planning as Global Frontier Project (2014M3A6A5060953), and by the Primary Research Program (16-12-N0101-33) of the Korea Electrotechnology Research Institute.References
- K. Ellmer, Nat. Photonics, 2012, 6, 809 CrossRef CAS.
- M. F. L. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535 CrossRef CAS PubMed.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270 CrossRef CAS PubMed.
- X. Ho and J. Wei, Materials, 2013, 6, 2155 CrossRef CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906 CrossRef CAS PubMed.
- R. Zhu, C.-H. Chung, K. C. Cha, j. W. Yang, Y. B. Zheng, H. Zhou, T.-B. Song, C.-C. Chen, P. S. Weiss, G. Li and Y. Yang, ACS Nano, 2011, 5, 9877 CrossRef CAS PubMed.
- S. Bae, H. Kim, Y. Lee, X. Xu, J.-S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. Kim, Y. I. Song, Y.-J. Kim, K. S. Kim, B. özyilmaz, J.-H. Ahn, B. H. Hong and S. Iijima, Nat. Nanotechnol., 2010, 5, 574 CrossRef CAS PubMed.
- J. T. Han, J. S. Kim, H. D. Jeong, H. J. Jeong, S. Y. Jeong and G.-W. Lee, ACS Nano, 2010, 4, 4551 CrossRef CAS PubMed.
- A. Kumar and C. Zhou, ACS Nano, 2010, 4, 11 CrossRef CAS PubMed.
- E. C. Garnett, W. Cai, J. J. Cha, F. Mahmood, S. T. Connor, M. G. Christoforo, Y. Cui, M. D. McGehee and M. L. Brongersma, Nat. Mater., 2011, 11, 241 CrossRef PubMed.
- L. Hu, H. S. Kim, J.-Y. Lee, P. Peumans and Y. Cui, ACS Nano, 2010, 4, 2955 CrossRef CAS PubMed.
- J.-Y. Lee, S. T. Connor, Y. Cui and P. Peumans, Nano Lett., 2008, 8, 689 CrossRef CAS PubMed.
- T.-B. Song, Y. Chen, C.-H. Chung, Y. M. Yang, B. Bob, H.-S. Duan, G. Li, K.-N. Tu, Y. Huang and Y. Yang, ACS Nano, 2014, 8, 2804 CrossRef CAS PubMed.
- J. Zhao, H. Sun, S. Dai, Y. Wang and J. Zhu, Nano Lett., 2011, 11, 4647 CrossRef CAS PubMed.
- H. H. Khaligh and I. A. Goldthorpe, Nanoscale Res. Lett., 2013, 8, 235 CrossRef PubMed.
- T. Kim, Y. W. Kim, H. S. Lee, H. Kim, W. S. Yang and K. S. Suh, Adv. Funct. Mater., 2013, 23, 1250 CrossRef CAS.
- J. S. Woo, J. T. Han, S. Jung, J. J. Jang, H. Y. Kim, H. J. Jeong, S. Y. Jeong, K.-J. Baeg and G.-W. Lee, Sci. Rep., 2014, 4, 4804 Search PubMed.
- P. Lee, J. Ham, J. Lee, S. Hong, S. Han, Y. D. Suh, S. E. Lee, J. Yeo, S. S. Lee, D. Lee and S. H. Ko, Adv. Funct. Mater., 2014, 24, 5671 CrossRef CAS.
- I. K. Moon, J. I. Kim, H. Lee, K. Hur, W. C. Kim and H. Lee, Sci. Rep., 2012, 3, 1112 Search PubMed.
- J. Liang, L. Li, K. Tong, Z. Ren, W. Hu, X. Niu, Y. Chen and Q. Pei, ACS Nano, 2014, 8, 1590 CrossRef CAS PubMed.
- Y. Ahn, Y. Jeong and Y. Lee, ACS Appl. Mater. Interfaces, 2012, 4, 6410 CAS.
- D. Lee, H. Lee, Y. Ahn, Y. Jeong, D.-Y. Lee and Y. Lee, Nanoscale, 2013, 5, 7750 RSC.
- Y. Jiang, J. Xi, Z. Wu, H. Dong, Z. Zhao, B. Jiao and X. Hou, Langmuir, 2015, 31, 4950 CrossRef CAS PubMed.
- Q. Huang, W. Shen, X. Fang, G. Chen, J. Guo, W. Xu, R. Tan and W. Song, RSC Adv., 2015, 5, 45836 RSC.
- J. Li, J. Liang, X. Jian, W. Hu, J. Li and Q. Pei, Macromol. Mater. Eng., 2014, 299, 1403 CrossRef CAS.
- J. T. Han, D. Kim, J. S. Kim, S. K. Seol, S. Y. Jeong, H. J. Jeong, W. S. Chang, G.-W. Lee and S. Jung, Appl. Phys. Lett., 2012, 100, 163120 CrossRef.
- K. Kabza, J. E. Gestwicki and J. McGrath, J. Chem. Educ., 2000, 77, 63 CrossRef CAS.
- E. Dujardin, T. W. Ebbesen, H. Hiura and K. Tanigaki, Science, 1994, 265, 1850 CAS.
- E. Dujardin, T. W. Ebbesen, A. Krishnan and M. M. J. Treacy, Adv. Mater., 1998, 10, 1472 CrossRef CAS.
- J. T. Han, J. S. Kim, S. G. Lee, H. Bong, H. J. Jeong, S. Y. Jeong, K. Cho and G.-W. Lee, J. Phys. Chem. C, 2011, 115, 22251 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17771a |
| This journal is © The Royal Society of Chemistry 2016 |