LaNiO3-nanorod/graphene composite as an efficient bi-functional catalyst for zinc–air batteries†
Jie Huab,
Qiunan Liub,
Ziwei Shib,
Liang Zhangb and
Hao Huang*ac
aState Key Laboratory of Metastable Materials Science & Technology, Yanshan University, Qinhuangdao, 066004, P. R. China. E-mail: huanghao@ysu.edu.cn; Fax: +86-335-8074545; Tel: +86-15369700375
bHebei Key Laboratory of Applied Chemistry, Department of Environment and Chemistry, Yanshan University, Qinhuangdao, 066004, P. R. China
cHenan Huanghe Whirlwind Co. Ltd., Changge, 461500, P. R. China
First published on 6th September 2016
Abstract
Developing low-cost catalysts for high-performance oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) is highly desirable. Herein, LaNiO3 nanorods supported on reduced graphene oxide (LNO-NR/RGO) were synthesized via a hydrothermal method, and characterized by XRD, SEM, TEM, XPS, TG and BET. The results show that the LaNiO3 nanorods have a perovskite structure and good dispersion behavior on the RGO sheets. The catalytic activity of the composite for ORR and OER has been studied by using a rotating disk electrode (RDE) technique. LNO-NR/RGO shows better oxygen electrode potential, a maximum cathodic current density of −4.26 mA cm−2 at 1600 rpm was obtained, and the ORR mainly favors a direct four electron pathway. Compared with pure LaNiO3 nanorods and commercial Pt/C, LNO-NR/RGO is more active for OER, a lower onset potential for OER and a bigger anodic current at the same applied potential are observed. The cycle performance and the stabilities of LNO-NR/RGO toward charge/discharge are significantly higher than those of commercial Pt/C in zinc–air batteries. Such excellent catalytic activity is attributed to the synergistic effect between LNO-NR and RGO along with the 1D conduction in the composite.
1. Introduction
Compared with other chemical-based batteries, metal–air batteries, as a result of their high theoretical energy densities, can contribute greatly in addressing the problems involved in the rapid growth of applications, particularly in the fields of electric and hybrid electric vehicles. The slow kinetics of ORR and OER at the cathode limits the efficiency of metal–air batteries.1–4 Platinum-based materials are known to be the most active electrocatalysts for the ORR and OER but suffer from prohibitive cost, susceptibility to methanol crossover and poor stability.5,6 Therefore, it is imperative to search for efficient and robust bifunctional electrocatalysts based on abundant nonprecious metals for widespread applications.Much effort has been put into developing cost-effective bifunctional catalysts such as transition metal oxides, which shows excellent ORR and OER activities.7–9 Particularly, metal oxides with a perovskite structure have received much attention as efficient electrode materials due to their relatively high electronic and ionic conductivity, such as CaMnO3−δ,10 and lanthanum-based perovskites.11–14 Nevertheless, the intrinsic electrical conductivity of most perovskites is quite poor due to their chemical structure and non-stoichiometric composition.15 Designing a one-dimensional (1D) nanostructured electrocatalysts, such as nanowires,16 nanotubes,17,18 and nanorods19,20 is an effective way to enhance the conductivity of oxides, and have attracted more and more attention in recent years. These complex structures are expected to offer more opportunities to tailor the physical chemical properties for fundamental studies and practical applications.
However, perovskite oxides typically have a reduced surface-to-weight ratio, easily agglomeration and are often characterized by low electronic conductivity,21 one of the key strategies is to use a conductive substrate to maximize the electroactive surface and enhance the electrical conductivity of the catalysts simultaneously. Graphene, a two-dimensional single-layer sheet of hexagonal carbon, shows high electrical conductivity, large surface area, good chemical and environmental stability, high mechanical strength, and structural flexibility, making it become an ideal substrate for supporting nanocrystal catalysts.22–24 Hey Woong Park and co-workers25 prepared porous nanorod La0.5Sr0.5Co0.8Fe0.2O3 using the electro-spinning method, which was mixed with nitrogen-doped reduced graphene oxide as a bifunctional catalyst for metal–air batteries. Ting Yang et al.15 synthetised CoMoO4 nanorods/reduced graphene oxide by a one-step facile wet chemical method, and measured its electrochemical performance of as anode material for lithium-ion batteries.
In the study of perovskite, LaNiO3 has a good bifunctional performance, which has caused wide attention. Jaka Sunarso et al. reported LaNiO3 catalysts show comparable performances in alkaline medium.26 Zhen zhen Du et al. reported Mg-substituted perovskites LaNi1−xMgxO3 are higher-performance catalysts for both the ORR and the OER in lithium–air batteries.27 In this paper, LaNiO3 nanorods (LNO-NR) supported on the reduced graphene oxide (RGO) were synthesized via a hydrothermal method. LaNiO3 nanorods with the longitudinal axes structure combined with RGO makes them have better dispersion, which possesses an anisotropic morphology that can improve mass and electron transport and catalyst utilization.28 The ORR and OER activity for LNO-NR/RGO in alkaline environment was investigated, and the corresponding reaction mechanism was evaluated. This report is the first to synthesize this sample, which proves to be an efficient catalyst for rechargeable Zn–air battery.
2. Experimental
2.1 Synthesis of LaNiO3-nanorod/RGO composites
In this study, reduced graphene oxide (RGO) materials were synthesized by a modified Hummer's method according to references.29–31 The following is a typical synthesis of LNO-NR/RGO. First, 48 mg RGO and 90 mL DI water was mixed to ultrasonic vibration for 1 h, then 20 mL of 0.1 M Ni(NO3)2 was mixed with 20 mL of 0.1 M La(NO3)3 solution were dissolved in 90 mL RGO/DI water suspension. Moreover, 1 M NaOH solution was added slowly to adjust the pH value to approximately 12. 0.0684 g CTAB as the template agent was added to the mixture solution, transferring the mixture into a 200 mL Teflon-line stainless steel autoclave, and heated at 180 °C for 24 h. At last, product was collected by filtration, washed with plenty of deionized water, and dried at 60 °C in an oven. The dried products were heated at 350 °C for 5 h in air to obtain a precursor powder and then at 650 °C for 4 h in vacuum to produce final samples. For comparisons, LaNiO3-nanorod (LNO-NR) was prepared through the same procedures, which was calcined at 650 °C for 4 h in air, and LaNiO3/RGO composites have been synthesized using the sol–gel technique according to references.322.2 Characterization
X-ray diffraction (XRD) was performed to examine the crystal structure of the sample using a Bede D1 X-ray diffractometer with Cu Ka radiation from 10° to 90° at a rate of 4° min−1. Furthermore, in order to examine the RGO weight content in the final product, thermogravimetric analysis (TG, 449C, NETZSCH, SELB, Germany) were carried out at a heating rate of 5 °C min−1 from 20 to 800 °C in air. The morphology of the composites was investigated by field-emission scanning electron microscopy (FESEM, Hitachi S-4800) and transmission electron microscopy (TEM, JEOL-2010) with an accelerating voltage of 200 kV. The binding energy of the elements was measured at room temperature by X-ray photoelectron spectroscopy (XPS, ESCALAB250) with a monochromated Al–Mg X-ray source (Al hν = 1486.6 eV; Mg hν = 1253.6 eV). The specific surface area was obtained from the results of N2 adsorption–desorption at 77 K (Quadrasorb SI, Quantachrome) using the Brunauere–Emmete–Teller (BET) method. Prior to measurements, the samples were degassed at 300 °C overnight under vacuum. Micropore volume from N2 sorption was calculated using the t-plot method. Pore size distributions data was calculated based on desorption data using the Barrette–Joynere–Halenda (BJH) method for mesopores and Horvathe–Kawazoe (HK) method for micropores.2.3 Electrochemical measurements
The inks were prepared by mixing 10 mg composites (the ratio of catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetylene black was 1
acetylene black was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 25 μL of Nafion solution (5 wt%), and 5 mL of isopropanol. Then, the ink was sonicated and dispersed for 30 min. A total of 25 μL ink was dropped to the glassy carbon working electrode (5 mm in diameter). The electrochemical characteristics and the activity of the composites were evaluated mainly by RDE techniques by using a Pine electrochemical system (AFMSRX rotator and AFCBP1 bipotentiostat), a platinum foil and Hg/HgO electrode serving as counter and reference electrodes, respectively. The CV measurements with a scan rate of 50 mV s−1 from −0.9 to 0.4 V was conducted in O2 saturated 0.1 M KOH solutions. The ORR in O2 saturated 0.1 M KOH solution at different rotation rates (400–1600 rpm) was used to evaluate the number of electron transferred (n) with a scan rate of 5 mV s−1 from −1 to 0.2 V. The OER performance was studied in N2 saturated electrolyte from 0 to 1 V at 1600 rpm. The n was determined using the Koutecky–Levich (K–L) equation (eqn (1))
2), 25 μL of Nafion solution (5 wt%), and 5 mL of isopropanol. Then, the ink was sonicated and dispersed for 30 min. A total of 25 μL ink was dropped to the glassy carbon working electrode (5 mm in diameter). The electrochemical characteristics and the activity of the composites were evaluated mainly by RDE techniques by using a Pine electrochemical system (AFMSRX rotator and AFCBP1 bipotentiostat), a platinum foil and Hg/HgO electrode serving as counter and reference electrodes, respectively. The CV measurements with a scan rate of 50 mV s−1 from −0.9 to 0.4 V was conducted in O2 saturated 0.1 M KOH solutions. The ORR in O2 saturated 0.1 M KOH solution at different rotation rates (400–1600 rpm) was used to evaluate the number of electron transferred (n) with a scan rate of 5 mV s−1 from −1 to 0.2 V. The OER performance was studied in N2 saturated electrolyte from 0 to 1 V at 1600 rpm. The n was determined using the Koutecky–Levich (K–L) equation (eqn (1))| id−1 = idl−1 + ik−1 = (Bω1/2)−1 + ik−1 | (1) |
| B = 0.62nFCo(Do)2/3ν−1/6 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 C mol−1), Co is O2 volume concentration in 0.1 M KOH (1.14 × 10−6 mol cm−3), ν is the kinematic viscosity of the electrolyte (0.01 cm2 s−1), and Do is the diffusion coefficient of O2 in 0.1 M KOH (1.73 × 10−5 cm2 s−1).33
500 C mol−1), Co is O2 volume concentration in 0.1 M KOH (1.14 × 10−6 mol cm−3), ν is the kinematic viscosity of the electrolyte (0.01 cm2 s−1), and Do is the diffusion coefficient of O2 in 0.1 M KOH (1.73 × 10−5 cm2 s−1).33
2.4 Fabrication and characterization of the zinc–air battery
In order to investigate the performance of catalysts in realistic metal–air battery, a homemade zinc–air battery was prepared and tested using a Neware Battery test instrument (CT-3008W-5V5Ma-S4, ShenZhen Neware Instrument Company). The cathode was made as follows: a certain amount of catalyst, acetylene black as well as 1 wt% poly tetrafluoroethylene (PTFE) were mixed to form homogeneous slurry, which was coated onto the nickel foam current collector (1 cm × 1 cm) using a blade. Subsequently, the air electrode was dried at 60 °C in air for 24 h to remove residual moisture. The final loading of active composition was 10.1 mg, which calculated based on the mass of catalyst and acetylene black in the cathode electrodes. The Ni-foam with the back side covered by the gas diffusion layer (a proofed breathable film) with 4 MPa to prevent the electrolyte from leaking out and supply air flow channels as well as reaction sites in the cathode of zinc–air batteries. A polished zinc plate as an anode, 6.0 M KOH was used as the electrolyte. Rechargeable single-cell zinc–air battery testing: the charge/discharge cycling was tested by the recurrent galvanic method using an applied current of 25 mA with each cycle consisting of 30 min of discharge followed by 30 min of charge.The AC impedance spectra were also measured on an electrochemical workstation (832C, Shanghai CH Instrument Company) at 20 °C with a frequency range between 1 MHz to 0.1 Hz, while the signal amplitude was 10 mV.
3. Results and discussions
3.1 XRD spectra analysis
The crystal structures of the samples were characterized via XRD, as shown in Fig. 1. The LNO-NR sample is well agrees with the characteristic peaks of perovskite phase with no other impurity. Several peaks located at 2θ = 32.914, 46.796 and 58.763, which can be indexed to the (110), (202), and (122) crystalline planes, respectively, based on the standard LaNiO3 (PDF 34-1181). Compared with LNO-NR, the main diffraction peaks of LNO-NR/RGO composites are similar, but small amount of NiO are observed in LNO-NR/RGO samples. As shown in Fig. 1, LNO/RGO nanoparticle sample is no longer the rhombohedral perovskite but the tetragonal structure of layered La2NiO4 (PDF 33-0712), which is consisted by LaNiO3 and LaO. This is due to the presence of RGO affect the decomposition of nitrates, the surface of graphene contains many oxygen groups, and the oxidation state of the metal ions is increased. Especially, when LNO/RGO nanocomposites are prepared by sol–gel method, the decomposition of nitrate in the precursor was incomplete, and it makes parts of trivalent metal ions become two valence.343.2 SEM-TEM analysis
As shown in Fig. 2a, the as-prepared RGO have gauze-shaped wrinkles and folds structure, which may be caused by oxygenic functional group and the resultant defects during the preparation of graphene oxide. And the as prepared RGO is about 3 μm in lateral sizes and an average thickness of 1.5 nm (Fig. S1a and b†).30 Its surface has some oxygenous groups such as carboxyl and carbonyl group (Fig. S2†). Fig. 2b shows the SEM image of pure LNO-NR samples, which indicates the aggregation of LaNiO3-nanorods in free RGO. From Fig. 2c and d, LNO-NR is well dispersed throughout the sheets of RGO without obvious aggregation. Thus the specific surface area of the catalyst is improved and the effect of electro catalysis is also increased. From Fig. 2d, it can be seen that the nanorods grow on the graphene sheet layer, which indicates that it can be used as the separation of graphene and prevents graphene layers from rebonding. The composite of these two substances, embodies the synergistic effect of LNO-NR and RGO. It can be seen from the Fig. 2e, La2NiO4 nanoparticles are uniformly distributed on the surface of RGO, and its particle size is 30–50 nm. The TEM image (Fig. 2f) exhibits LNO-NR/RGO nanorods with the diameter about 10 nm and length of 100 nm. In addition, from the HRTEM (Fig. 2g), the lattice fringes as observed is about 0.272 nm, corresponding to the (110) planes of LaNiO3 crystals. The selected area electron diffraction patterns (SAED) in Fig. 2h distinctly indicate some concentric rings arising from the diffractions of the (200), (110) and (122) planes of LNO-NR/RGO composite, which is in accordance with XRD analysis.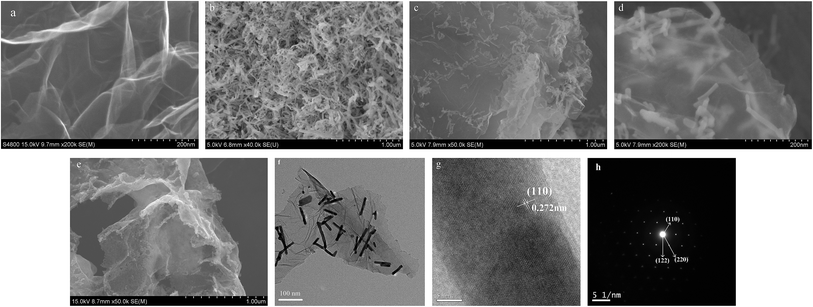 | ||
| Fig. 2 (a and b) SEM image of RGO and LNO-NR, (c and d) low and high magnification SEM images of LNO-NR/RGO, (e) SEM image of La2NiO4/RGO, (f) TEM, (g) HRTEM image and (h) SAED pattern of LNO-NR/RGO. | ||
3.3 XPS analysis
In order to obtain the cation oxidation state and the surface chemical composition of the LNO-NR and LNO-NR/RGO, X-ray photoelectron spectroscopy (XPS) measurements were conducted. The overall spectrum in Fig. 3a shows the La3d, Ni2p, O1s and C1s peaks in the LNO-NR/RGO. In the high resolution spectra of Ni in LNO-NR and LNO-NR/RGO (Fig. 3b), the main peak at ∼852.5 eV is comparable to the Ni2+ species. The binding energy at ∼856.3 eV corresponds to the spin–orbit characteristic of Ni3+. The ratio of Ni2+/Ni3+ is 1.11 for LNO-NR/RGO and 0.81 for LNO-NR (Fig. 3b). Since there is a similarity of peak position and a difference of Ni2+/Ni3+ ratio for Ni XPS spectra, it can be seen that the introduction of RGO takes effect on the Ni cationic distribution of LNO. This result is consistent with XRD analysis. It had been reported that the transition metals with mixed valences could provide donor–acceptor chemisorption sites for the reversible adsorption of oxygen and realize high electric conductivity for electron hopping between cations with different valences.35 Moreover, the interconnected RGO sheets, as perfectly conducting channels, can also improve the electron transport rate from semiconducting catalyst to the external circuit. These suggest that the structure of the mixed valent Ni–O compound coupled with RGO gives potentially high catalytic activity for LNO-NR/RGO hybrid.34 As shown in Fig. 3c, the observed spectra are successfully deconvoluted into three component curves (A, B, and C). The low binding energy peak at 527.55–528.3 eV (A) is ascribed to the lattice oxygen in the lanthanum and nickel oxides (β oxygen). The additional high binding energy peaks at 529.5–530.05 eV (B) and 530.8–531 eV (C) originate from hydroxide species and chemisorbed oxygen (α oxygen).33 It can been seen from Fig. 3c, the C (α oxygen) peak area of the LNO-NR/RGO is apparently higher than that of the LNO-NR, the adsorbed oxygen is related to oxygen oxide defects, and usually accompanied by the formation of B element valence state, which indicating oxygen vacancies increased with the introduction of RGO, it can result in superior ORR catalytic performance of LNO-NR/RGO. Besides the B (hydroxide species) peak area of the LNO-NR/RGO is also higher than that of the LNO-NR, the content of surface hydroxyl (–OH) at the perovskite type oxide catalyst surface also affects the speed of oxygen evolution reaction, because the hydroxyl (–OH) is involved in the generation of hydrogen peroxide O–O bond. With the increased concentration of catalyst surface hydroxyl (–OH), the generation speed of HOO– is become more faster, and the performance of oxygen oxidation reaction (OER) catalytic is improved.36 Therefore, the increase of the concentration of α oxygen and hydroxyl group (–OH) on the LNO-NR/RGO have a perfact catalytic effect for the ORR and OER.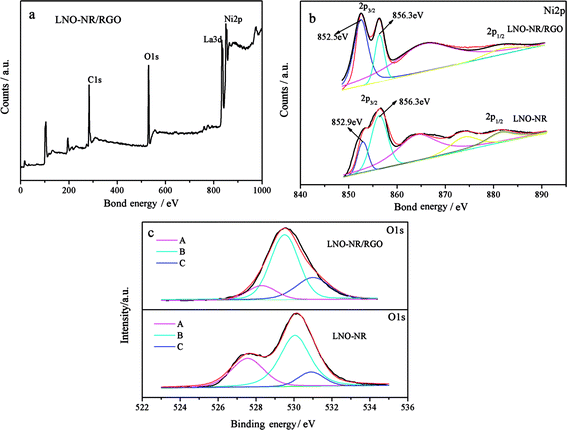 | ||
| Fig. 3 (a) XPS spectrum surveys scan, (b) high resolution Ni2p and (c) O1s spectra for LNO-NR and LNO-NR/RGO. | ||
3.4 TG and BET analysis
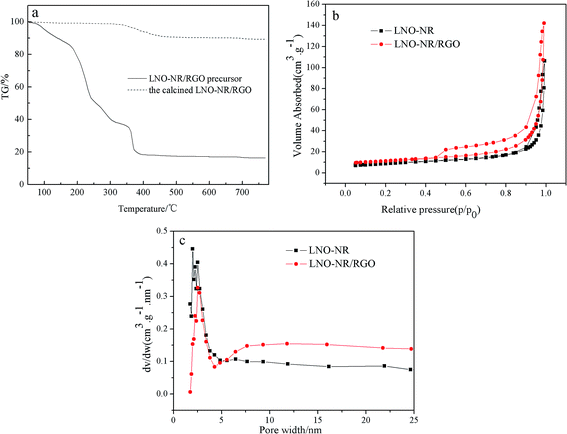
In order to indicate the RGO weight content in the final product, the TG curves were analyzed, and the results are shown in Fig. 4a. In the TG curve of the precursor, the weight loss before 200 °C was attributed to the evaporation of free water, and the rapid weight loss of approximately 220 °C was due to the lost process of crystal water. At 330 °C to 400 °C, the sharp descendant curve resulted from the decomposition of nitrate. However, the TG curve of the calcined LNO-NR/RGO sample depicted little weight loss before 350 °C. It only showed a big exothermic peak at 350 °C to 420 °C, which was due to the exothermic reactions of RGO in the air. Notably, the weight loss of calcined LNO-NR/RGO sample reached 10%, which indicate the RGO weight content in the final product is about 10 wt%.Nitrogen sorption measurements are carried out to investigate the pore structure and size distribution. Fig. 4b and c shows the nitrogen adsorption desorption isotherms and pore size distribution diagrams for LNO-NR and LNO-NR/RGO. The N2 sorption isotherms of the LNO-NR and LNO-NR/RGO demonstrate a typical H3 type isotherm with the hysteresis loop in the P/P0 range of 0.44–1.0 (Fig. 4b), illustrating the characteristic of mesoporous structure. It can be seen in Fig. 4b, LNO-NR/RGO have a larger adsorption capacity of nitrogen than LNO-NR, and the specific surface areas of LNO-NR/RGO and LNO-NR are 38.013 and 30.612 m2 g−1, respectively. The pore size for LNO-NR is ranged from 1.9–2.5 nm, while that of LNO-NR/RGO main center at 2.5 nm (Fig. 4c). It can be seen from the pore size distribution curves, addition of RGO has significantly increased the quantities of mesopores, therefore, increasing the mesopores volume (0.220 cm3 g−1 for LNO-NR/RGO and 0.164 cm3 g−1 for LNO-NR) and the average pore diameter (13.865 nm for LNO-NR/RGO and 10.782 nm for LNO-NR). Because LaNiO3-nanorods are dispersed well on the surface of RGO, while reducing the aggregation, larger pore diameters and pore volumes can is beneficial for the transport of O2 and electrolyte, and may also contribute the high surface exposure of active sites for ORR and OER.37
3.5 Electrocatalytic activity
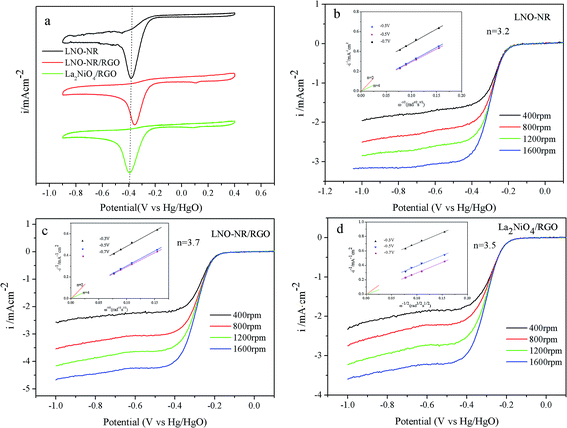
The catalytic activities of the LNO-NR, LNO-NR/RGO, La2NiO4/RGO toward ORR were first examined by cyclic voltammograms (CV) in O2 saturated 0.1 M KOH at room temperature (Fig. 5a). The ORR onset potential and peak potential of LNO-NR/RGO are −0.185 and −0.379 V vs. Hg/HgO, respectively. These values are more positive than those of LNO-NR (onset of −0.207 V and peak of −0.384 V) and La2NiO4/RGO (onset of −0.203 V and peak of −0.406 V). As for the LaNiO3 nanorods, the transmission of electrons is limited to a special dimension, which avoids the transmission resistance of electrons at the grain boundaries, thus the conductivity is better than La2NiO4 particles. At the same time, the LaNiO3 nanorods are dispersed on the RGO surface. RGO is not only as supports for LaNiO3 nanorods to increase the active sites, but also as electronic conductive channels, thereby improving the mass transfer process. Thus, the ORR peak potential of LNO-NR/RGO is more positive than the other two samples. To investigate the kinetics of LNO-NR, LNO-NR/RGO, La2NiO4/RGO, the rotating-disk measurements with different rotation rates were carried out (Fig. 5b–d). The corresponding electron transfer number (n) was calculated from the slopes of Koutecky–Levich plots at −0.3, −0.5 and −0.7 V (inset of Fig. 5b–d). As a result, the electron transfer numbers of LNO-NR, La2NiO4/RGO were calculated to be 3.2 and 3.5, respectively. While, the LNO-NR/RGO shows the higher electron transfer number, up to 3.7, which favors a four electron ORR pathway produces.38As for the ORR curves, the limiting current density is −4.26 mA cm−2 for LNO-NR/RGO, approximately 1.4 times greater than that of LNO-NR (−3.04 mA cm−2) (Fig. 6). Compared with La2NiO4/RGO, LNO-NR/RGO has a slightly more positive half-wave potential (−0.3175 V) than La2NiO4/RGO (−0.319 V), with slightly positive onset potential and relatively high limiting current density. It reveals that LNO-NR/RGO is a more effective catalyst for ORR. As for the one-dimensional nanorods materials, the transmission of electrons is limited to a special dimension, which avoids the transmission resistance of electrons at the grain boundaries, thus improving the conductivity. But the electrical conductivity of the composite is still weaker than that of commercial Pt/C, so it indeed exhibits inferior ORR activity relative to Pt/C catalyst. Besides the ORR activity, excellent OER activities are particularly critical for bifunctional catalysts. Fig. 6 also shows OER polarization curves of above samples in N2-saturated 0.1 M KOH with a sweeping rate of 5 mV s−1. Among all the samples, LNO-NR/RGO exhibited the best OER performance, with a high current density of 33.51 mA cm−2 at 1 V and an onset potential of 586 mV, while commercial Pt/C has obvious poor OER activities. It is convenient to estimate the overall electrocatalytic activity and the reversibility of LNO-NR/RGO as an oxygen electrode in alkaline electrolyte, by the variance matrices Δ(Ej=10 mA − E1/2) between ORR and OER.35 The value is about 1.0775 V for LNO-NR/RGO whereas it is 1.105 V for La2NiO4/RGO, 1.183 V for LNO-NR and 1.18046 V for commercial Pt/C. It has been demonstrated that LNO-NR/RGO is potentially favorable to be a bi-functional oxygen catalyst.
 | ||
| Fig. 6 Comparison among the LSV curves of ORR activity, ORR/OER mechanism, and OER activity of LNO-NR, LNO-NR/RGO, La2NiO4/RGO and commercial Pt/C at 1600 rpm. | ||
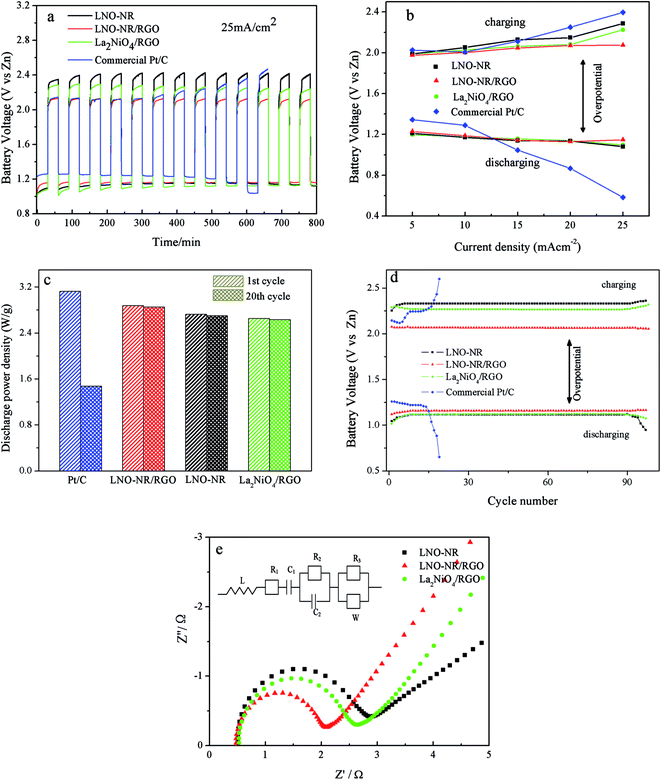
In order to better understand the activity of the bi-functional catalyst, the Zn–air battery performance was constructed and evaluated by discharging and charging (30 min in each state) at a current density 25 mA cm−2. As shown in Fig. 7a, LNO-NR/RGO shows a small potential gap (between charge and discharge) of 0.97 V, while LNO-NR, La2NiO4/RGO, and Pt/C shows potential gap of 1.28 V, 1.143 V and 1.434 V, respectively. The excellent single-cell performance demonstrated by LNO-NR/RGO here clearly shows its cost competitiveness over precious metal-based commercial catalysts. The catalytic activity of LNO-NR/RGO at different current densities is also examined using the galvanostatic method in Fig. 7b. During discharge, the catalytic behavior of LNO-NR/RGO is similar to that of LNO-NR and La2NiO4/RGO and lower than commercial Pt/C in the low-current range, but it is better than that of Pt/C in the high-current range. During charge, LNO-NR/RGO outperforms greatly than Pt/C in the high-current range with lower overpotential, which is mainly attributed to the poor OER activity of Pt/C. Fig. 7c is the discharge power densities of different samples for 1st and 20th cycle. In comparison with the cathode catalyzed by Pt/C, the LNO-NR/RGO based cathode shows a better discharge capability for zinc–air battery. After 20 cycles, the LNO-NR/RGO based cathode can still maintain a relatively high discharge power density of 2.85 W g−1, but only 1.47 W g−1 is retained for commercial Pt/C, which indicates that the capacity retention of the LNO-NR/RGO is better than commercial Pt/C. The cycling durability is shown in Fig. 7d. It shows that LNO-NR/RGO has the best performance among all of above samples and the lowest decline. At the initial cycle, the charge and discharge potential of LNO-NR/RGO is 1.096 and 2.073 V, as well as after 8 cycles, the values change to 1.136 and 2.068 V, respectively, they keep stable until 97 cycles. The round trip efficiency is about 58% at 97th cycle (59.7% for the 1st cycle) for LNO-NR/RGO, 44.3% (51% for the 1st cycle) for La2NiO4/RGO, 40.1% for LNO-NR (45.5% for the 1st cycle), whereas 24% at 19th cycle (59% for the 1st cycle) for commercial Pt/C. This result indicates that the combination of LNO-NR and RGO remarkably reduced the discharge–charge voltage gap and improved round-trip efficiency. The rapid decline for Pt/C may be attributed to the particle dissolution and aggregation in the alkaline electrolyte upon long-time electrocatalysis.39 The synergistic effect between LNO-NR and RGO in the composite can inhibit the particulate agglomeration of LaNiO3-nanorod and the re-bonding of RGO sheet, therefore an improved durability is achieved.
The electrochemical impedance spectroscopy (EIS) technique (Fig. 7e) is used to provide further insight on the kinetics of electrode reactions, which is fitted using the following equivalent circuit: R1C1(R2C2W)(R3W)L. The Nyquist plots (Zreal vs. Zim) of LNO-NR, LNO-NR/RGO and La2NiO4/RGO are almost similar in form with an arc in the high-frequency region (corresponding to charge transfer resistance, Rct) and a quasi-sloping line in the low-frequency region (corresponding to mass transfer resistance). L is the equivalent inductance, C1 is the limit capacitance, and R1 is a combinational resistance of ionic resistance of electrolyte, intrinsic resistance of substrate, and contact resistance at the active material/current collector interface. A major difference of the impedance plots is the semicircle in the high frequency range, which corresponds to the charge transfer resistance R2 caused by the faradaic reactions and the double-layer capacitance C2 on the grain surface. The smaller R2 values of LNO-NR/RGO compared with LNO-NR and La2NiO4/RGO is a strong indication of the improvement in ORR/OER kinetics. Fitting of the impedance data by an equivalent circuit is presented in Table 1. The slope of the curve is called the Warburg resistance W and R3, which is the result of the frequency dependence of ion diffusion/transport in the electrolyte to the electrode surface. It can be seen in the Fig. 7e, the slope of LNO-NR is the least in the low frequency region. This is because the introduction of graphene increases the active site of catalyst, thereby increasing the three-phase reaction interface,32 and it is easier for electrolyte ion transfer.
| Sample | L | R1 | C1 | R2 | C2 | R3 | W |
|---|---|---|---|---|---|---|---|
| LNO-NR | 6.172 × 10−8 | 0.6569 | 0.0015 | 2.281 | 2.416 × 10−6 | 7.24 | 0.0254 |
| LNO-NR/RGO | 5.198 × 10−8 | 0.4041 | 0.0026 | 1.085 | 3.943 × 10−6 | 5.07 | 0.0351 |
| La2NiO4/RGO | 5.48 × 10−8 | 0.4493 | 0.0013 | 1.474 | 2.522 × 10−6 | 13.18 | 0.0314 |
4. Conclusions
In summary, a novel hybrid electrocatalyst of LaNiO3 nanorods which were dispersed on reduction graphene oxide is synthesized via a hydrothermal process. ORR/OER results show that the composites have a better catalytic activity compared with LaNiO3 nanorods and commercial Pt/C, because one dimensional LaNiO3 nanorods are well dispersed on the RGO surface, and the active site of the catalyst is increased. The cycle performance and stability of this hybrid catalyst were evaluated by preparing air electrodes, which is outperformed than commercial Pt/C benchmark in alkaline condition. The unique structural, morphological, and electrocatalytic properties of the LNO-NR/RGO make this hybrid material show excellent performance and it is a promising candidate for zinc–air battery applications.Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (No. 51402253), the Natural Science Foundation of Hebei Province (No. B2016203172), and the China Postdoctoral Science Foundation (No. 2015M582191).Notes and references
- S. Ratso, I. Kruusenberg, M. Vikkisk, U. Joost, E. Shulga, I. Kink, T. Kallio and K. Tammeveski, Carbon, 2014, 73, 361 CrossRef CAS.
- C. F. Chen, G. King, R. M. Dickerson, P. A. Papin, S. Gupta, W. R. Kellogg and G. Wu, Nano Energy, 2015, 13, 423 CrossRef CAS.
- Y. Y. Liang, Y. G. Li, H. L. Wang and H. J. Dai, J. Am. Chem. Soc., 2013, 135, 201 Search PubMed.
- C. Jin, X. C. Cao, F. L. Lu, Z. R. Yang and R. Z. Yang, Int. J. Hydrogen Energy, 2013, 38, 10389 CrossRef CAS.
- H. Yin, C. Z. Zhang, F. Liu and Y. L. Hou, Adv. Funct. Mater., 2014, 24, 2930 CrossRef CAS.
- K. Lee, M. S. Ahmed and S. Jeon, J. Power Sources, 2015, 288, 261 CrossRef CAS.
- P. H. Benhangi, A. Alfantazi and E. Gyenge, Electrochim. Acta, 2014, 123, 42 CrossRef CAS.
- W. N. Yan, Z. R. Yang, W. Y. Bian and R. Z. Yang, Carbon, 2015, 92, 74 CrossRef CAS.
- D. K. Lim, H. N. Im, J. Kim and S. J. Song, J. Phys. Chem. Solids, 2013, 74, 115 CrossRef CAS.
- J. Du, T. R. Zhang, F. Y. Cheng, W. S. Chu, Z. Y. Wu and J. Chen, Inorg. Chem., 2014, 53, 9106 CrossRef CAS PubMed.
- F. L. Lu, J. Sui, J. M. Su, C. Jin, M. Shen and R. Z. Yang, J. Power Sources, 2014, 271, 55 CrossRef CAS.
- W. G. Hardin, J. T. Mefford, D. A. Slanac, B. B. Patel, X. Q. Wang, S. Dai, X. Zhao, R. S. Ruoff, K. P. Johnston and K. J. Stevenson, Chem. Mater., 2014, 26, 3368 CrossRef CAS.
- M. Prabu, P. Ramakrishnan, P. Ganesan, A. Manthiram and S. Shanmugam, Nano Energy, 2015, 15, 92 CrossRef CAS.
- J. I. Jung, H. Y. Jeong, J. S. Lee, M. G. Kim and J. Cho, Angew. Chem., 2014, 126, 4670 CrossRef.
- T. Yang, H. N. Zhang, Y. Z. luo, L. Mei, D. Guo, Q. H. Li and T. H. Wang, Electrochim. Acta, 2015, 158, 327 CrossRef CAS.
- D. C. Higgins, R. Y. Wang, M. A. Hoque, P. Zamani, S. Abureden and Z. W. Chen, Nano Energy, 2014, 10, 135 CrossRef CAS.
- Q. F. Yi, H. Chu, M. X. Tang, Z. Yang, Q. H. Chen and X. P. Liu, J. Electroanal. Chem., 2015, 739, 178 CrossRef CAS.
- P. F. Li, J. K. Zhang, Q. L. Yu, J. S. Qiao, Z. H. Wang, D. Rooney, W. Sun and K. N. Sun, Electrochim. Acta, 2015, 165, 78 CrossRef CAS.
- Y. J. Xu, A. Tsou, Y. Fu, J. Wang, J. H. Tian and R. Z. Yang, Electrochim. Acta, 2015, 174, 551 CrossRef CAS.
- B. Sun, J. Q. Zhang, P. Munroe, H. J. Ahn and G. X. Wang, Electrochem. Commun., 2013, 31, 88 CrossRef CAS.
- H. Zhao, C. Chen, D. J. Chen, M. Saccoccio, J. Wang, Y. Gao, T. H. Wan and F. Ciucci, Carbon, 2015, 90, 122 CrossRef CAS.
- Z. L. Wang, D. Xu, J. J. Xu, L. L. Zhang and X. B. Zhang, Adv. Funct. Mater., 2012, 22, 3699 CrossRef CAS.
- L. Shi, Z. Y. Chu, Y. Liu, W. Q. Jin and N. P. Xu, Adv. Funct. Mater., 2014, 24, 7032 CrossRef CAS.
- R. S. Edwards and K. S. Coleman, Nanoscale, 2013, 5, 38 RSC.
- H. W. Park, D. U. Leea, P. Zamani, M. H. Seo, L. F. Nazar and Z. W. Chen, Nano Energy, 2014, 10, 192 CrossRef CAS.
- J. Sunarso, A. A. J. Torriero, W. Zhou, P. C. Howlett and M. Forsyth, J. Phys. Chem. C, 2012, 116, 5827 CAS.
- Z. Z. Du, P. Yang, L. Wang, Y. H. Lu, J. B. Goodenough, J. Zhang and D. W. Zhang, J. Power Sources, 2014, 265, 91 CrossRef CAS.
- S. J. Peng, L. L. Li, Y. X. Hu, M. Srinivasan, F. Y. Cheng, J. Chen and S. Ramakrishna, ACS Nano, 2015, 9, 1945 CrossRef CAS PubMed.
- S. Ratso, I. Kruusenberg, M. Vikkisk, U. Joost, E. Shulga, I. Kink, T. Kallio and K. Tammeveski, Carbon, 2014, 73, 361 CrossRef CAS.
- J. Hu, J. H. Ma, L. N. Wang and H. Huang, J. Alloys Compd., 2014, 583, 539 CrossRef CAS.
- D. Phihusut, J. D. Ocon, B. Jeong, J. W. Kim, J. K. Lee and J. Lee, Electrochim. Acta, 2014, 140, 404 CrossRef CAS.
- J. Hu, L. N. Wang, L. N. Shi and H. Huang, Electrochim. Acta, 2015, 161, 115 CrossRef CAS.
- X. X. Zhang, Q. Q. Xiao, Y. X. Zhang, X. Jiang, Z. Y. Yang, Y. F. Xue, Y. M. Yan and K. N. Sun, J. Phys. Chem. C, 2014, 118, 20229 CAS.
- Q. Liu, J. T. Jin and J. Y. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 5002 CAS.
- L. Wang, C. Lin, D. K. Huang, F. X. Zhang, M. K. Wang and J. Jin, ACS Appl. Mater. Interfaces, 2014, 6, 10172 CAS.
- W. G. Hardin, D. A. Slanac, X. Q. Wang, S. Dai, K. P. Johnston and K. J. Stevenson, J. Phys. Chem. Lett., 2013, 4, 1254 CrossRef CAS PubMed.
- W. Zhou and J. Sunarso, J. Phys. Chem. Lett., 2013, 4, 2982 CrossRef CAS.
- M. Prabu, P. Ramakrishnan and S. Shanmugam, Electrochem. Commun., 2014, 41, 59 CrossRef CAS.
- Y. Y. Liang, Y. G. Li, H. L. Wang, J. G. Zhou, J. Wang, T. Regier and H. J. Dai, Nat. Mater., 2011, 10, 780 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16610e |
| This journal is © The Royal Society of Chemistry 2016 |