Preparation of carbon nanotubes/porous polyimide composites for effective adsorption of 2,4-dichlorophenol
Abstract
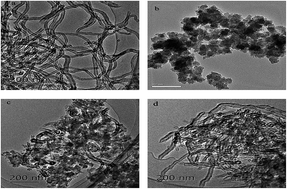
The novel carbon nanotubes (CNT)/porous polyimide (PI) composites were prepared via blending and were characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), transmission electron microscopy (TEM) and nitrogen adsorption–desorption techniques. The feasibility of CNT, CNT/PI (3/1) and CNT/PI (1/1) to remove 2,4-dichlorophenol (2,4-DCP) from an aqueous solution was examined. The influence of different parameters such as pH, ionic strength, contact time and temperature has been carried out. The results showed that PI was successfully combined with CNT and formed CNT/PI composites with higher adsorption capacity and shorter equilibrium time. The adsorption capability at 298 K of CNT, CNT/PI (3/1) and CNT/PI (1/1) composites were 330.0 mg g−1, 412.5 mg g−1 and 506 mg g−1, respectively. Their kinetic experimental data were better fitted by a pseudo-second-order model, and their adsorption isotherms could be represented by the Freundlich isotherm model perfectly. A thermodynamic study showed that the adsorption of 2,4-DCP on them was spontaneous and endothermic. Discoveries in this study suggest that the CNT/PI composites are promising adsorbents for removal of 2,4-DCP from aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...