Dipeptide-bonded stationary phases for hydrophilic interaction liquid chromatography†
Magdalena Skoczylas,
Szymon Bocian and
Bogusław Buszewski*
Chair of Environmental Chemistry and Bioanalytics, Faculty of Chemistry, Nicolaus Copernicus University, Gagarin 7, 87-100 Torun, Poland. E-mail: bbusz@chem.umk.pl; Fax: +48 56 611 4837; Tel: +48 56 611 4308
First published on 29th September 2016
Abstract
Dipeptides of glycine and alanine were directly synthesized onto a silica surface using an established procedure based on the fundamental principles of the Merrifield method. The peptide-silica stationary phases were prepared via a two-stage reaction including initial modification of the silica with γ-aminopropyltrimethoxysilane and subsequent bonding of the protected amino acids (alanine and glycine). The surface properties of the stationary phases, before and after chemical modification, were characterized by different physicochemical techniques. The resulting data provided information about the structure of the bonded ligands, the heterogeneity of the peptide layers, and a potential specification for the hydrophilic chromatography mechanism. Thereafter, the obtained packing materials were applied as stationary phases for the analysis of nucleic bases and nucleosides in HILIC mode. The application and comparison of the packing materials with a commercially available column was also performed. Both peptide-silica adsorbents were also compared in terms of their retention and separation selectivity of nucleosides and nucleic bases.
1. Introduction
Hydrophilic interaction liquid chromatography (HILIC) is a powerful separation system dedicated to the analysis of highly polar compounds.1–3 HILIC provides conditions for resolving problems which occur in normal phase (NP) and reversed phase (RP) liquid chromatography (LC). The mixed-mode mechanism in the HILIC system causes difficulties with proper interpretation of the phenomena occurring in the chromatographic system. It is still under investigation and discussion.4–6 Nevertheless, the HILIC mode is an essential method in the fields of life science connected with genomics, proteomics, metabolomics etc. Therefore, the application of highly selective and efficient stationary phases (SPs) for HILIC is prominent.4,6Peptide-silica stationary phases7–9 are noteworthy materials characterized by their specific properties and the multitude of possible combinations of their structures. The first application of peptide-bonded stationary phases in liquid chromatography was conducted by Losse and Kuntze.10 Unfortunately, the applied stationary phase was not made on silica gel, and was unstable under high pressure.10 Grushka and Scott11 were the initiators of chemical bonding of peptides to a silica gel surface. The original stationary phases with bonded tripeptides were synthesized using Merrifield’s reactor.7–9,11 After the introduction of peptide-based modification novel separation materials frequently called bio-inspired stationary phases were developed.12–17 For this purpose, the “graft-to” method including antecedent synthesis of peptide molecules by solution-phase processes12,13,15,16 as well as click chemistry14,17 were investigated. The novel materials were applied inter alia for the separation of D,L-amino acids, isomeric dipeptides, PTH-amino acids,9 bioactive polar compounds (nucleobases and nucleosides),15,16 steroids, sulfur-based drug molecules,16 the traditional Chinese medicine (TCM) Rheum Palmatum L. in an off-line 2D-RP/RPLC,14 and finally in a molecular-shape selective HPLC for polycyclic aromatic hydrocarbons (PAHs).12 Nevertheless, stationary phases with bonded peptides do not possess as much hydrophilic character as the poly(2-hydroxyethyl aspartamide)-coated silica, which was the primary peptide-material applied in HILIC mode. This stationary phase has been commercially available since 1990 as PolyHYDROXYETHYL A.1,18
The solid-phase peptide synthesis method introduced by Merrifield19 is based on subsequent connection of amino acids to a solid support. The application of protecting groups and an activator with carboxyl moieties is required.19,20 To the best of our knowledge, there is no report on the application of the fundamental principles of the Merrifield method in the synthesis of peptides onto a silica surface, except with the use of an expensive Merrifield’s reactor. Furthermore, peptides are commonly synthesized by conventional solution phase methodologies, and then attached to the silica support as polypeptide chains.15,16 In contrast, the developed methodology avoids the labor-intensive and inefficient process of purification of intermediate products, due to the bonding of subsequent amino acids, according to the original concepts of solid-phase peptide synthesis.15,21
Therefore, the aim of this research is to illustrate the preparation strategy and further characterize the chromatographic properties of peptide-silica stationary phases in detail. Two materials were synthesized and characterized: amino-Gly and amino-Ala, which contain immobilized dipeptides of glycine and alanine, respectively. The structure of the chemically bonded ligands was confirmed by elemental analysis, thermogravimetric analysis, solid state 13C NMR and FTIR spectroscopy. This work also presents the application of the materials in HILIC mode for analysis of biologically significant compounds with different physicochemical properties. The influence of bonded peptide chains was investigated and compared with an amino-bonded material (which comprises a support for peptide-packing material synthesis) as well as with a commercial column. The quantitative description of the retention behavior of the analyzed compounds on the peptide-silica stationary phases was also demonstrated.
2. Experimental
2.1. Instruments, materials, and method
Detailed description of applied instruments, materials, and method is available in the ESI.†16,22,232.2. Synthesis procedure
The surface of bare silica was chemically modified with γ-aminopropyltrimethoxysilane under vacuum in a glass reactor, which provides complete insulation from the external environment. Synthesis conditions for the silica-amino were performed according to the methodology described earlier.24 The phases with covalently bonded dipeptides were prepared using amino-bonded silica as a support (Fig. S1, ESI†). The first step of the synthesis was to remove the physically adsorbed water by drying silanized silica gel for 12 h at a temperature of 80 °C ± 5 °C under reduced pressure. Further, the prepared support was bonded with the first C-terminal amino acid (with a protected amino group). For this purpose, 5.6 g of functionalized silica gel and 40 ml of a suspension of 6 g of Fmoc-Ala-OH and 3 g of DCCI (activator of carboxyl group) in anhydrous DMF and DCM were placed in a glass flask and heated to a temperature of 40 °C using an oil bath. The reaction was carried out for 24 h under reflux. The intermediate products were washed with toluene and methanol on a Schott funnel, in order to remove the excess reactants.The next stage of the synthesis was the deprotection of the bonded amino acids. The removal of the Fmoc groups was carried out using 50 ml of a 20% solution of piperidine in anhydrous DMF. The resulting suspension was stirred for 15 min, and then the product was filtered and washed with toluene and methanol consecutively. The prepared adsorbent was dried under vacuum at 60 °C overnight. The stationary phase comprising the first layer of alanine was further derivatized in the same manner as that described above. The developed method enables two stationary phases with dipeptide alanine (amino-Ala) and glycine (amino-Gly) chemically bonded to their surface to be obtained.
3. Results and discussion
3.1. Instrumental characterization
Dipeptide-bonded stationary phases were characterized by elemental analysis (Table S1, ESI†),25 solid state nuclear magnetic resonance spectroscopy (13C NMR) (Fig. S2, ESI†),26–30 Fourier transform infrared spectroscopy (FTIR) (Table S2, ESI†),16,22,24 and thermogravimetric analysis (TGA) (Fig. S3, ESI†).16,31–343.2. HILIC separation mechanism
In order to illustrate the chromatographic properties of the peptide-silica materials, retention plots of nucleic bases are presented in Fig. 1. These results exemplify the dependence of the logarithm of the retention factor of a solute (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k) on the volume fraction of water in a binary aqueous–organic mobile phase. The nucleic bases’ retention over the high-organic composition mobile phase decreased with increasing water content once the HILIC conditions were achieved. This is associated with the unification of the mobile phase and the water layer adsorbed onto the surface of the adsorbents. Thus, increased residence times of the solutes in the mobile phase and consequent reduction of their retention were noted. The HILIC behavior of bonded ligands is confirmed by the increase in retention times in comparison with when silica-amino material is used as a support for the synthesis (Fig. 1C).
k) on the volume fraction of water in a binary aqueous–organic mobile phase. The nucleic bases’ retention over the high-organic composition mobile phase decreased with increasing water content once the HILIC conditions were achieved. This is associated with the unification of the mobile phase and the water layer adsorbed onto the surface of the adsorbents. Thus, increased residence times of the solutes in the mobile phase and consequent reduction of their retention were noted. The HILIC behavior of bonded ligands is confirmed by the increase in retention times in comparison with when silica-amino material is used as a support for the synthesis (Fig. 1C).
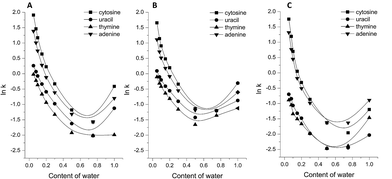 | ||
Fig. 1 Changes in nucleic base retention (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k) vs. water content in the mobile phase: (A) amino-Gly SP, (B) amino-Ala SP, (C) silica-amino SP. k) vs. water content in the mobile phase: (A) amino-Gly SP, (B) amino-Ala SP, (C) silica-amino SP. | ||
In the case of RP mode, the elution of nucleic bases in the void volume of the column was observed. This suggests that despite the presence of hydrophobic moieties such as organic chains and methyl groups, peptide-silica stationary phases also have hydrophilic properties. It should be emphasized that an increase in nucleic base retention under the pure-water conditions was noted. However, the elution of solutes was achieved around the void time, and separation with high selectivity was impossible. The peptide-silica stationary phases have similar properties which are confirmed by the approximate relations of their retention plots. Retention on the amino-Gly SP (Fig. 1A) is to some extent higher than the amino-Ala SP (Fig. 1B), especially under the HILIC conditions.
In order to compile the retention properties of the tested materials under conditions universally used in HILIC mode, the influence of the water content in the mobile phase comprising the buffer salt was also investigated. For this purpose, the volume percentage of water in the eluent was changed from 30% to 5% while the concentration and pH of the ammonium acetate buffer solution were maintained at 20 mM and pH = 6.51. The chromatographic behavior of nucleic bases on both dipeptide-silica stationary phases is shown in Fig. S4 in the ESI.† The results are in compliance with the dependence obtained from the mobile phase void of the buffer salt. Accordingly, the retention factors decreased with an increase of the water content, which is in accordance with the standard retention behavior of polar compounds in HILIC.
The investigation of the effect of the concentration of buffer solution in the mobile phase comprises an essential contribution for the retention mechanism studies. Hence, ammonium salt was chosen due to its relatively high solubility at high organic modifier levels which enables its compatibility with the conditions commonly used in HILIC. Ammonium acetate (NH4Ac) at different concentrations (5–20 mM) in the mobile phase was used in order to study the effect of salt concentration, and the pH was kept at 6.51. The retention of nucleosides and nucleic bases was studied with four different concentrations of the buffer solution in an acetonitrile–water mobile phase with the concentration of the organic modifier maintained at 92% and 90%, respectively. The plots of retention factor and concentration of ammonium acetate for both groups of compounds are shown in Fig. 2. In the case of nucleic bases (Fig. 2A and B), the retention factors slightly increased or remained practically the same with increasing salt concentration in the mobile phase. This relationship indicates that the partitioning phenomena comprise the predominant retention mechanism of this type of compound. Therefore, the influence of ionic interactions in the retention of nucleic bases was weak.15,35–37 On the other hand, the retention of nucleosides (Fig. 2C and D) in the range of low salt concentration was slightly increased, while it decreased with higher ionic strengths of the mobile phase. Moreover, in the case of compounds with the highest retention times (guanosine, cytidine, and N2-methylguanosine) a more explicit decrease in the retention over the range of higher salt concentrations was observed. The decrease in the retention with increasing ionic strength of the mobile phase arises from the presence of ionic interactions between the solute and immobilized ligands on the surface of the stationary phases. This result indicates the influence of ionic interactions in the overall retention of this type of polar compound and justifies the multivariate retention mechanism in HILIC mode.15,38,39
The investigation into the general tendency of the relationship between the retention factor and the content of water in the mobile phase provides common knowledge about the chromatographic properties of columns dedicated for HILIC mode. The quantitative description of retention behavior in HILIC on peptide-silica SPs was performed with utilization of multiple regression analysis. For this purpose, the analysis based on the HPLC equation (eqn (1)) proposed by Lu et al.40,41 was investigated.
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = a + b k = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2O + cCH2O CH2O + cCH2O
| (1) |
The applied model allows precise characterization of retention dependency over a wide concentration of solvents in the mobile phase. Moreover, the assumptions of this model are based on the theory, not only the mathematical calculation. Consequently, the parameter a is associated with the volume of the solute as well as the interaction energy between the solute and stationary and mobile phases. Parameter b is the coefficient of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2O in the retention equation and is related to the direct analyte–stationary phase interaction. Finally, the coefficient c is related to the interaction energy between the solute and solvents.35,42
CH2O in the retention equation and is related to the direct analyte–stationary phase interaction. Finally, the coefficient c is related to the interaction energy between the solute and solvents.35,42
Multiple regression analysis was performed for the retention of nucleic bases and nucleosides on the amino-Gly and amino-Ala SPs. In the case of nucleic bases, the retention data from the water content of the mobile phase in the range presented in Fig. 1 was considered. The retention mechanism of nucleosides on the synthesized packing materials was investigated in the mobile phase composition characteristically used for HILIC mode (the volume fraction of water was in the range of 0.05–0.5). The regression results for amino-Gly SP and amino-Ala SP based on eqn (1) are shown in Table 1.
| Nucleic bases | Amino-Gly | Amino-Ala | ||||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | R2 | a | b | c | R2 | |
| Thymine | −0.27 ± 0.14 | −0.16 ± 0.04 | −4.68 ± 0.37 | 0.9997 | −1.01 ± 0.03 | −0.33 ± 0.01 | −1.68 ± 0.09 | 0.9999 |
| Uracil | −0.44 ± 0.14 | −0.29 ± 0.04 | −2.97 ± 0.31 | 0.9992 | −1.85 ± 0.23 | −0.65 ± 0.09 | 0.96 ± 0.26 | 0.9670 |
| Adenine | −2.70 ± 0.33 | −1.38 ± 0.13 | 1.88 ± 0.38 | 0.9860 | −2.28 ± 0.23 | −1.12 ± 0.09 | 1.66 ± 0.27 | 0.9883 |
| Cytosine | −2.77 ± 0.34 | −1.57 ± 0.13 | 2.33 ± 0.40 | 0.9862 | −2.54 ± 0.24 | −1.38 ± 0.09 | 2.22 ± 0.27 | 0.9909 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Nucleosides | ||||||||
| N2-Methylguanosine | −2.10 ± 0.31 | −1.72 ± 0.11 | −0.61 ± 0.55 | 0.9985 | −1.56 ± 0.17 | −1.32 ± 0.06 | −1.22 ± 0.30 | 0.9997 |
| 7-Methylguanosine | −1.96 ± 0.29 | −1.32 ± 0.10 | −0.63 ± 0.51 | 0.9979 | −1.68 ± 0.14 | −1.10 ± 0.05 | −0.66 ± 0.24 | 0.9994 |
| Adenosine | −0.68 ± 0.25 | −0.78 ± 0.09 | −3.15 ± 0.45 | 0.9983 | −0.29 ± 0.06 | −0.51 ± 0.02 | −3.17 ± 0.11 | 0.9998 |
| Guanosine | −1.79 ± 0.23 | −1.75 ± 0.08 | −0.80 ± 0.41 | 0.9992 | −1.37 ± 0.15 | −1.36 ± 0.05 | −1.31 ± 0.26 | 0.9996 |
| 1-Methylguanosine | −1.00 ± 0.26 | −1.08 ± 0.09 | −2.51 ± 0.45 | 0.9986 | −0.46 ± 0.10 | −0.71 ± 0.04 | −2.99 ± 0.18 | 0.9997 |
| Cytidine | −1.03 ± 0.31 | −1.29 ± 0.11 | −2.12 ± 0.55 | 0.9983 | −0.59 ± 0.16 | −0.94 ± 0.05 | −2.57 ± 0.27 | 0.9994 |
| 2-Deoxythymidine | −0.91 ± 0.19 | −0.56 ± 0.07 | −3.16 ± 0.34 | 0.9987 | −0.54 ± 0.03 | −0.32 ± 0.01 | −2.97 ± 0.06 | 0.9999 |
| 1-Methylinosine | −0.71 ± 0.34 | −0.80 ± 0.12 | −3.11 ± 0.60 | 0.9971 | −0.17 ± 0.10 | −0.42 ± 0.04 | −3.44 ± 0.18 | 0.9995 |
| 1-Methyladenosine | −0.84 ± 0.25 | −0.71 ± 0.09 | −3.04 ± 0.45 | 0.9981 | −0.19 ± 0.05 | −0.35 ± 0.02 | −3.55 ± 0.09 | 0.9999 |
| Uridine | −0.51 ± 0.24 | −0.69 ± 0.08 | −2.93 ± 0.42 | 0.9981 | −0.18 ± 0.11 | −0.40 ± 0.04 | −3.07 ± 0.19 | 0.9994 |
| Pseudouridine | −0.14 ± 0.26 | −0.88 ± 0.09 | −3.21 ± 0.45 | 0.9985 | 0.22 ± 0.14 | −0.52 ± 0.05 | −3.59 ± 0.26 | 0.9992 |
The comparison of collected data reveals better results for regression analysis in the case of nucleosides. According to the data in Table 1, the regression coefficients were all about 0.999 for the amino-Ala SP and 0.997 for the amino-Gly SP. Considering coefficient b in the retention equation, which corresponds to the direct analyte–stationary phase interaction, about two times higher values for amino-Gly SP were observed. This suggests and confirms the more polar character of the stationary phase with chemically bonded dipeptides of glycine.
Moreover, the results of the multiple regression analysis and linear solvation strength model43 (e.g. the linear dependency of the logarithm of the retention factor on the concentration of the stronger elution solvent) were not consistent. Thus, the retention mechanism on the synthesized peptide-silica packing materials is not governed by a pure partitioning mechanism. The other interactions such as adsorption, hydrogen bonding, electrostatic interactions, ion-exchange etc. also contributed to retention on the peptide-silica stationary phases. The quantitative description of retention in HILIC comprises also a significant part of the prediction and optimization of a particular chromatographic system.
3.3. Separation of polar compounds under HILIC conditions
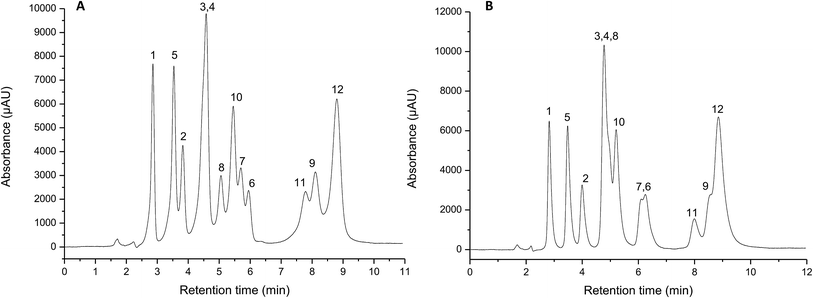
The peptide-silica stationary phases were used as polar packing material in HILIC mode for the analysis of biologically significant compounds. As seen in Fig. 3A and B, both stationary phases allow the separation of a mixture of nucleic bases with good selectivity. Due to low solubility at neutral pH, guanine was eliminated from the mixture. It should be noted that gradient elution was required, because in an isocratic run the thymine and uracil do not separate, while the remaining compounds eluted much later in the form of asymmetric and broadened peaks. The application of a linear gradient consisting of the direct reduction amount (98–85/80%) of acetonitrile in the mobile phase allows for the achievement of a complete separation of the four nucleic bases. The amino-Gly SP needs to have a lower content of acetonitrile (80% ACN) in the eluent, due to its stronger hydrophilic properties. The polar analytes showed stronger interactions with the surface of the stationary phase. Therefore, it was necessary to use a mobile phase richer in the component having a higher elution strength (water).Additionally, the separation of a mixture of nucleic bases on the silica-amino SP was performed (Fig. 3C). In comparison with the peptide-bonded packing materials, the selectivity was lower, and resulted in incomplete separation under the same gradient regime.
To demonstrate the applicability of the synthesized stationary phases, separation of nucleosides was also performed. For this purpose, peptide-silica SPs, silica-amino material, as well as a commercial Luna-HILIC column were used. As shown in Fig. 4A and B, the selectivity of separation on the two peptide-silica SPs was different. In the case of the amino-Gly SP, the separation was almost complete, in contrast to the amino-Ala, for which the chromatogram shows a partial separation. As expected, the observed effects were caused by an additional methyl group in the alanine molecule, which gives slightly hydrophobic properties to the surface of the stationary phase. Therefore, hydrophilic analytes were retained less on the stationary phase with the chemically bonded dipeptide of alanine. These interactions cause a reduction in the retention times of the individual compounds. The time of separation on the amino-Gly SP was extended by more than 10 min, due to the greater affinity of the polar analytes to the polar stationary phase. The retention mechanism on the peptide-silica SPs was multivariate. On one hand, the partition equilibrium typical of HILIC was observed, but on the other, hydrogen bonding and electrostatic interactions also play an essential part in retaining hydrophilic compounds. It can be emphasized that good resolution was obtained for the nucleoside isomers 7-methylguanosine and 1-methylguanosine, which are baseline separated.
In connection with proving the significance of peptide-ligands, the silica-amino SP was used. According to Fig. 4C, the complete separation of nucleosides was not achieved. It can be assumed that the appearance of bonded hydrophilic dipeptide chains leads to an increase in selectivity. It should also be noted that the heterogeneity of the surface of the synthesized packing materials has an effect on the retention. The presence of dipeptide chains, as well as a monolayer of not-extended amino acid groups, and the unbonded amino moieties of the support create a specific retention mechanism.44,45 Additionally, the application of the commercial Luna-HILIC column (Fig. 4D) also presents an inferior resolution. Although the retention mechanism on this column is similar to that of the packing materials of interest, the selectivity is lower in comparison with the peptide-silica SPs (especially for amino-Gly SP). It should be emphasized that the comparison of the prepared packing materials with the commercial column was performed under conditions particularly advantageous to the peptide-silica materials. Consequently, the separation of nucleosides on the Luna-HILIC column may be performed with appropriate resolution. However, the application of identical conditions during the study enabled the compliance of the tested materials. Particular parameters of the separations and consequently the Purnell equation, which include retention factor (k), the number of theoretical plates (N), resolution (Rs), selectivity (α), and asymmetry factor (fAs) are listed in Table S3 in the ESI.† The efficiency of the tested columns was obtained up to 6012 and 8530 theoretical plates per 125 mm column length for amino-Ala and amino-Gly, respectively. In the case of the amino-Gly SP, the obtained peaks are more symmetrical, with an asymmetry factor in the range of 0.93–1.13. In contrast, the peaks obtained for the amino-Ala SP were more asymmetric. Additionally, the asymmetry factor was not calculated for peaks that were not separated to the baseline.
The application of a pure hydro-organic mobile phase in the separation of nucleosides had the intention of allowing a direct comparison of the two dipeptide-silica SPs. Operation under these conditions indicates the original properties of the prepared stationary phases. Nevertheless, investigation under HILIC conditions allows in some cases for the improvement of separation during the use of buffer solution. In the case of amino-Ala SP (Fig. 5A), a higher selectivity together with a reduction in the retention time by half was observed due to the application of a mobile phase comprising 20 mM ammonium acetate. In contrast, the elevated ionic strength of the mobile phase causes inferior resolution which is connected with poor selectivity of nucleoside separation on the amino-Gly SP (Fig. 5B). An explanation may be found by considering the effect of the concentration of the buffer solution in the mobile phase. The decrease in the retention in the range of the higher salt concentration is slightly larger for the amino-Ala SP. This may suggest that ionic interactions play a significant role in the retention mechanism. In the case of amino-Gly SP the modification of the ionic strength of the mobile phase causes unification and a loss of specificity of interactions between the solute and peptide-ligands localized on the surface of the packing material. The modification of the mobile phase by the buffer salt also causes changes in the elution order. It should be mentioned that the improvement in the efficiency which is explicitly connected with the optimization of the packing methodology would cause the baseline separation.
The tested stationary phases may also be compared in terms of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k parameters. Hydrophilic solutes which included analyzed nucleosides and nucleic bases were chosen. The discussed correlations between the two columns are shown in Fig. 6. From both groups of compounds the slope parameters are equal to approximately 1.2, which proves that the retention on amino-Gly SP is stronger than on the amino-Ala SP. It confirms that the material with bonded glycine is more polar and thus provides a stronger retention in HILIC. The presence of methyl groups in amino-Ala SP reduces the retention of polar compounds.
k parameters. Hydrophilic solutes which included analyzed nucleosides and nucleic bases were chosen. The discussed correlations between the two columns are shown in Fig. 6. From both groups of compounds the slope parameters are equal to approximately 1.2, which proves that the retention on amino-Gly SP is stronger than on the amino-Ala SP. It confirms that the material with bonded glycine is more polar and thus provides a stronger retention in HILIC. The presence of methyl groups in amino-Ala SP reduces the retention of polar compounds.
Nevertheless, both phases display similarities in their retention properties. For both packing materials relatively high determination coefficients were obtained (r2 = 0.9850 for nucleosides, and r2 = 0.9978 for nucleic bases). The peptide bond, which is present in the structure of the two stationary phases, provided hydrogen bonding and dipole–dipole interactions, which may have a strong effect on the analyzed solutes. Moreover, the amino groups of the peptide-silica materials, as the pKa = 10, can be protonated under the conditions of this study. This also creates electrostatic interactions between the negatively charged analyte molecules and the cations localized on the surface of the stationary phases. Furthermore, the methyl group presence in the alanine molecule may bring about weak hydrophobic interactions.
The intercepts of the correlation plots are in the range 0.070–0.096. The reason for this may be due to the differences in the physical column parameters e.g. the coverage density of the peptide ligand, and the heterogeneity of the chemically bonded moieties.
3.4. Reproducibility and stability
The amino-Gly and amino-Ala columns demonstrated high reproducibility of retention time and column stability. The separation of nucleic bases under gradient conditions was repeated three times for each peptide-silica column to evaluate the repeatability of the gradient runs.The relative standard deviation (RSD) of the retention times was in the range 0.02–0.05% for amino-Gly SP and 0.03–0.05% for amino-Ala SP. The equilibration time of the columns was 6 min. In conclusion, the results indicate that the synthesized peptide-silica stationary phases are suitable columns in terms of repeatability and reproducibility of the gradient analysis.
Moreover, under the conditions of continuous injection in a HILIC system an applicable reproducibility was noted. The peptide-silica columns have been used for about 10 months (running for over 800 h) in our laboratory. The relative standard deviations of the retention times calculated based on the results obtained for the first time using the peptide-packing materials and after the mentioned period are in the range 0.02–0.09% and 0.01–0.03% for stationary phases with chemically bonded dipeptides of glycine and alanine, respectively. Further application of the synthesized stationary phases for the following 6 months (overall 16 months of usage) with similar intensity did not change the stability of the prepared materials. The RSDs are comparable with data after 10 months of use and their values are in the range 0.02–0.09% for amino-Gly SP and 0.01–0.06% for amino-Ala SP. Furthermore, the efficiency of the peptide-silica stationary phases is maintained at a constant level. The relative standard deviations of the number of theoretical plates for the two mentioned periods (10 months and 16 months) are in the range 0.09–0.22% and 0.07–0.21% for amino-Gly SP and 0.05–0.13% and 0.08–0.34% for amino-Ala SP. Consequently, long term usage did not result in obvious changes to the retention time and column efficiency.
4. Conclusions
The procedure based on the fundamental principles of the Merrifield method comprised an effective and convenient way to synthesize peptide-silica stationary phases. Accordingly, in this simple manner it was possible to synthesize a peptide chain directly on the solid support (silica gel), however, the surface of the resulting stationary phase was heterogeneous.The obtained materials were investigated using instrumental analysis. The synthesized peptide-silica stationary phases demonstrated typical chromatographic behavior for HILIC mode. Chromatographic evaluation under HILIC conditions revealed that the retention mechanism of the peptide-silica materials was provided by a partition mechanism as well as other interactions e.g. electrostatic interactions, hydrogen bonding, ion-exchange etc.
The obtained stationary phases were successfully applied in a HILIC system for the analysis of polar compounds. In the case of nucleosides, amino-Gly SP provides higher selectivity in relation to the amino-Ala material. The methyl group in the alanine molecule caused increased hydrophobicity of the surface of the stationary phase. On the other hand, both stationary phases allow the separation of a mixture of nucleic bases with good selectivity and efficiency. It has to be emphasized that despite the slight difference in the structure of the two stationary phases, the differences were observed in the retention of individual compounds. Therefore, designing the sequence and thereby the structure of the peptide-silica adsorbents enabled us to obtain highly selective stationary phases for liquid chromatography which we intend to further investigate.
Acknowledgements
This work was supported by grants from the Ministry of Science and Higher Education “Iuventus Plus” no. IP2014 003673 for the period 2015–2017, and NCN (MAESTRO-6) no. 2014/14/A/ST4/00641 for the period 2015–2018. The authors are grateful to Akzo Nobel (Bohus, Sweden) for their kind donation of the Kromasil 100 silica gel used in this study. This publication is dedicated to our faithful friend Professor Eli Grushka.References
- A. J. Alpert, J. Chromatogr. A, 1990, 499, 177–196 CrossRef CAS PubMed.
- P. J. Boersema, S. Mohammed and A. J. R. Heck, Anal. Bioanal. Chem., 2008, 391, 151–159 CrossRef CAS PubMed.
- V. V. Tolstikov and O. Fiehn, Anal. Biochem., 2002, 301, 298–307 CrossRef CAS PubMed.
- P. Jandera, Anal. Chim. Acta, 2011, 692, 1–25 CrossRef CAS PubMed.
- M. R. Gama, R. G. d. Costa, C. H. Collins and C. B. G. Bottoli, Trends Anal. Chem., 2012, 402, 231–247 Search PubMed.
- B. Buszewski and S. Noga, Anal. Bioanal. Chem., 2012, 402, 231–247 CrossRef CAS PubMed.
- G. W.-K. Fong and E. Grushka, Anal. Chem., 1978, 50, 1154–1161 CrossRef CAS.
- G. W.-K. Fong and E. Grushka, J. Chromatogr. A, 1977, 142, 299–309 CrossRef CAS PubMed.
- E. J. Kikta and E. Grushka, J. Chromatogr. A, 1977, 135, 367–376 CrossRef CAS PubMed.
- G. Losse and K. Kuntze, Z. Chem., 1970, 10, 22–29 CrossRef CAS.
- E. Grushka and R. P. W. Scott, Anal. Chem., 1973, 45, 1626–1633 CrossRef CAS PubMed.
- A. Shundo, T. Sakurai, M. Takafuji, S. Nagaoka and H. Ihara, J. Chromatogr. A, 2005, 1073, 169–174 CrossRef CAS PubMed.
- S. Ray, M. Takafuji and H. Ihara, Anal. Methods, 2014, 6, 7674–7680 RSC.
- M. Xue, H. Huang, Y. Ke, C. Chu, Y. Jin and X. Liang, J. Chromatogr. A, 2009, 1216, 8623–8629 CrossRef CAS PubMed.
- S. Ray, M. Takafuji and H. Ihara, Analyst, 2012, 137, 4907–4909 RSC.
- S. Ray, M. Takafuji and H. Ihara, J. Chromatogr. A, 2012, 1266, 43–52 CrossRef CAS PubMed.
- A. Shen, X. Li, X. Dong, J. Wei, Z. Guo and X. Liang, J. Chromatogr. A, 2013, 1314, 63–69 CrossRef CAS PubMed.
- N. P. Dinh, T. Jonsson and K. Irgum, J. Chromatogr. A, 2013, 1320, 33–47 CrossRef CAS PubMed.
- R. B. Merrifield, J. Am. Chem. Soc., 1963, 85, 2149–2154 CrossRef CAS.
- G. B. Fields and R. L. Noble, Int. J. Pept. Protein Res., 1990, 35, 161–214 CrossRef CAS PubMed.
- M. Bodanszky and A. Bodanszky, The practice of peptide synthesis, Springer, 1984, pp. 1–282 Search PubMed.
- S. Bocian, A. Nowaczyk and B. Buszewski, Anal. Bioanal. Chem., 2012, 404, 731–740 CrossRef CAS PubMed.
- B. Buszewski, R. Gadzała-Kopciuch, R. Kaliszan, M. Markuszewski, M. T. Matyska and J. J. Pesek, Chromatographia, 1998, 48, 615–622 CAS.
- B. Buszewski, M. Jezierska, M. Wełniak and R. Kaliszan, J. Chromatogr. A, 1999, 845, 433–445 CrossRef CAS.
- G. E. Berendsen and L. deGalan, J. Chromatogr. A, 1980, 196, 21–37 CrossRef CAS.
- B. Buszewski, R. M. Gadzała-Kopciuch, M. Markuszewski and R. Kaliszan, Anal. Chem., 1997, 69, 3277–3284 CrossRef CAS.
- B. Buszewski, J. Schmid, K. Albert and E. Bayer, J. Chromatogr. A, 1991, 552, 415–427 CrossRef CAS PubMed.
- K. Albert and E. Bayer, J. Chromatogr. A, 1991, 544, 345–370 CrossRef CAS.
- B. Buszewski, Z. Suprynowicz, P. Staszczuk, K. Albert, B. Pfeiderer and E. Bayer, J. Chromatogr. A, 1990, 499, 305–316 CrossRef CAS.
- R. M. Silverstein, F. X. Webster and D. J. Kiemle, Spectrometric identification of organic compounds, New York, 7th edn, 2005 Search PubMed.
- T. M. H. Costa, M. R. Gallas, E. V. Benvenutti and J. A. H. d. Jornada, J. Non-Cryst. Solids, 1997, 220, 195–201 CrossRef CAS.
- Y.-L. Liu, W.-L. Wei, K.-Y. Hsu and W.-H. Ho, Thermochim. Acta, 2004, 412, 139–147 CrossRef CAS.
- R. Mueller, H. K. Kammler, K. Wegner and S. E. Pratsinis, Langmuir, 2003, 19, 160–165 CrossRef CAS.
- C. P. Jaroniec, R. K. Gilpin and M. Jaroniec, J. Phys. Chem. B, 1997, 101, 6861–6866 CrossRef CAS.
- Z. Guo, Y. Jin, T. Liang, Y. Liu, Q. Xu, X. Liang and A. Lei, J. Chromatogr. A, 2009, 1216, 257–263 CrossRef CAS PubMed.
- T. Czajkowska and M. Jaroniec, J. Liq. Chromatogr. Relat. Technol., 1996, 19, 2829–2841 CrossRef CAS.
- B. Buszewski, Z. Safaei and S. Studzińska, Open Chem., 2015, 13, 1286–1292 Search PubMed.
- A. Shen, Z. Guo, X. Cai, X. Xue and X. Liang, J. Chromatogr. A, 2012, 1228, 175–182 CrossRef CAS PubMed.
- L. Xu, R. Peng, X. Guan, W. Tang, X. Liu and H. Zhang, Anal. Bioanal. Chem., 2013, 405, 8311–8318 CrossRef CAS PubMed.
- P. Z. Lu, X. M. Lu, X. Z. Li and Y. K. Zhang, Chin. Sci. Bull., 1982, 19, 1175 Search PubMed.
- P. Z. Lu, X. M. Lu, X. Z. Li and Y. K. Zhang, Chin. Sci. Bull., 1982, 21, 1307 Search PubMed.
- G. Jin, Z. Guo, F. Zhang, X. Xue, Y. Jin and X. Liang, Talanta, 2008, 76, 522–527 CrossRef CAS PubMed.
- P. Česla, N. Vaňková, J. Křenková and J. Fisher, J. Chromatogr. A, 2016, 1438, 179–188 CrossRef PubMed.
- T. Czajkowska, I. Hrabovsky, B. Buszewski, R. K. Gilpin and M. Jaroniec, J. Chromatogr. A, 1995, 691, 217–224 CrossRef CAS.
- T. Czajkowska and M. Jaroniec, J. Chromatogr. A, 1997, 779, 29–71 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental detail, characterization, and additional results. See DOI: 10.1039/c6ra17704b |
| This journal is © The Royal Society of Chemistry 2016 |