Indole-BODIPY: a “turn-on” chemosensor for Hg2+ with application in live cell imaging†
Navdeep Kaura,
Paramjit Kaur*a,
Gaurav Bhatiab,
Kamaljit Singh*a and
Jatinder Singhb
aDepartment of Chemistry, UGC-Centre of Advanced Studies-II, Guru Nanak Dev University, Amritsar-143005, India. E-mail: paramjit19in@yahoo.co.in; kamaljit.chem@gndu.ac.in
bDepartment of Molecular Biology and Biochemistry, Guru Nanak Dev University, Amritsar-143005, India
First published on 26th August 2016
Abstract
A new BODIPY, 2-(bis(1-methyl-1H-indol-3-yl)methyl)-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene bearing bis(1-methyl-1H-indol-3-yl)methyl unit at the β-pyrrolic position acting as a ‘turn-on’ chemosensor for Hg2+ in solution as well as in HeLa cells, is synthesized. The ‘off’ state of the probe is proposed to be a result of a photoinduced electron transfer (PET) process which gets inhibited in the presence of Hg2+ ions, leading to the ‘on’ state. We propose that the detection of Hg2+ ions proceeds via the interaction of the π-electron density of the electron rich indolic units of the BODIPY group with that of the acidic Hg2+ ions, restricting the PET. The sensing protocols are established by various spectroscopic/electrochemical/DFT studies.
Introduction
The development of chemical sensors based upon emission and absorption changes has witnessed considerable advancement in recent years. However, the emission based sensors have attained an edge over the absorption based sensors owing to their ample use in biochemical labelling, electroluminescent materials, laser dyes, photonic materials etc. Among these, 4-bora-3a,4a-diaza-s-indacene (BODIPY) based probes have emerged as versatile fluorescent probes owing to their characteristic photophysical properties such as strong absorption and emission with excellent quantum yields.1–3 Moreover, owing to the thermal stability and photochemical inertness, these have emerged as important components in a variety of applications such as photovoltaic devices, non-linear optical (NLO) materials, near infrared (NIR) absorbing dyes, etc.4–9 Importantly, the electronic properties of BODIPY based molecular probes could be tuned by functionalisation of BODIPY core at meso-position, as well as pyrrolic at α and β positions, which has efficiently been achieved by various research groups.4,10–15 Suitable substitution of the BODIPY probes, such as having electron donating groups on the pyrrole ring(s) show significant modulation of their electronic properties.14–16 In continuation of our interest in the development of molecular sensing probes, we previously reported synthesis and sensing of probes based on BODIPY fluorophore appended with a dithia-dioxa-aza crown ether and ferrocene at meso-position, for Pd2+ and Hg2+/Cr3+, respectively.17,18 As the functionalization of the β-pyrrolic position of the BODIPY is comparatively less studied in comparison to α-position functionalization,4,19 herein, we report the synthesis of a BODIPY dye with bis(1-methyl-1H-indol-3-yl)methyl unit at β-pyrrolic position. The molecular electrostatic potential (MEP) calculated for bis(1-methyl-1H-indol-3-yl)methane using Gaussian 09 suite of programs20 (Fig. 1), suggested electron rich indole units to interact with an acidic guest.21 Indeed, the dye detects Hg2+ ions through such interaction, which in fact constitutes one of the rare mechanisms among the reported sensing probes22–30 for Hg2+ ions. Detection of mercury becomes important as all of its three forms i.e. elemental, inorganic and organic are highly toxic, and have lethal effects on the living beings caused by prenatal brain damage, cognitive and motion disorder, vision and hearing loss, central nervous and endocrine system disruptions etc.31–37 The atmospheric contamination caused by the release of mercury through gold production, coal combustion and industrial waste, is also an important issue of concern.38Experimental section
Chemicals
Solvents (analytical grade) used for the analytical work were purchased from Thomas Baker, while the ones used for the synthetic work were of synthesis grade. The metal salts and N-methyl indole were purchased from Sigma-Aldrich and used as such. Stock solutions (0.1 M) of perchlorate salts of Li+, Na+, Mg2+, Ca2+, Pb2+, Ba2+, Mn2+, Ni2+, Cu2+, Hg2+, Cr3+, Fe3+ and nitrate salts of K+, Ag+, Zn2+, Cd2+, La3+, Ce3+, Pr3+, Sm3+, Gd3+, Nd3+, Tb3+ ions were prepared in double distilled water, whereas solution of 1 (5 × 10−6 M) was prepared in acetonitrile.Instrumentation
IR spectra were recorded on a Perkin Elmer Fourier-Transform spectrophotometer in the range 400–4000 cm−1 in KBr pellets. 1H (500 MHz), 19F (282 MHz) and 13C NMR (125 MHz) spectra were recorded in CDCl3 and CD3CN on AVANCE III Bruker spectrometer. Tetramethylsilane (TMS) was used as an internal standard for 1H and 13C NMR spectra, whereas, trifluoroacetic acid (TFA) was used as an external reference standard (δ: −76 ppm) for 19F NMR spectrum. Data are reported as follows: chemical shift in ppm (δ), multiplicity (s = singlet, d = doublet), integration, coupling constant J (Hz) and assignment. The NMR titrations (19F: 282 MHz 1H: 300 MHz) were performed on JEOL-FT NMR-AL at 300 MHz. Mass spectra were recorded on a microTOF-Q II 10356 high resolution mass spectrometer (HRMS). The electrochemical studies were done on a CHI 660C electrochemical workstation with a conventional three-electrode configuration consisting of a glassy carbon and a counter electrode and a Ag/AgCl electrode as the reference. The experiments were carried out at room temperature in a 10−4 M solution of the sample in anhydrous (dried over P2O5) acetonitrile containing 0.01 M n-tetrabutylammonium hexafluorophosphate (TBAPF6) as the supporting electrolyte. Deoxygenation of the solutions was achieved by purging dry nitrogen gas for 10 min and the working electrode was cleaned after each run. The voltammograms were recorded with a scan rate of 100 mV s−1. Emission studies were performed on Perkin Elmer LS 55 Fluorescence Spectrometer with excitation slit width as 15 nm and emission slit width as 10 nm with off emission correction mode. UV-visible absorption spectra were recorded on a SHIMADZU 1601 PC spectrophotometer, with a quartz cuvette (path length, 1 cm). The cell holder of the spectrophotometer was maintained at 25 °C for consistency in the recordings. Melting points were determined in open capillaries and are uncorrected. The cell imaging was carried out on the NIKON A1R confocal microscope.Quantum yield calculations
The fluorescence quantum yields were measured using 9,10-diphenylanthracene as standard having quantum yield of 0.95 in cyclohexane39 using the following equation:| ϕu = ϕsFu(1 − 10−AsLs)nu2/(1 − 10−AuLu)FsAuns2 |
Computational details
All theoretical calculations were carried out by using the Gaussian 09 suite of programs.20 The molecular geometries of the chromophores were optimized at the DFT method employing the hybrid B3LYP functional. 6-31G* basis set was used for C, H, B, F, and N atoms whereas SDD basis set for Hg2+. The same model chemistry was used for the calculation of the properties of the chromophores. The first 15 excited states were calculated by using time-dependent density functional theory (TD-DFT calculations). The molecular orbital contours were plotted using Gauss view 5.0.9.Cell culture and confocal cell imaging
Human cervix adenocarcinoma HeLa cell line was obtained from NCCS, Pune, India and maintained on Dulbecco's Modified Eagle's Medium (DMEM) supplemented with streptomycin (100 U mL−1), gentamycin (100 μg mL−1), 10% FBS (Sigma-Aldrich) at 37 °C and humid environment containing 5% CO2.For imaging, HeLa cells were cultured on 18 mm glass coverslips in 12 well plates at a concentration of 3 × 104 cells per well and allowed to grow for 48 hours (till 70–80% confluence). Experiments to assess Hg2+ uptake were performed in the same media supplemented with different concentrations of Hg(ClO4)2 (20 and 30 μM) for 2 hours 30 min. Cells were washed twice with phosphate buffered saline (PBS) before incubating with 5 μM 1 in PBS for 30 min at 25 °C. The cells were again washed twice with PBS before imaging. Confocal imaging of HeLa cells was achieved using NIKON A1R confocal laser scanning microscope using diode laser with excitation at 570 nm. Imaging was carried out with Plan Apo 40× objective lens.
Procedure for synthesis and characterisation of 1
 | ||
| Scheme 1 Synthetic route for the synthesis of compound 1: (a) (i) TFA, DCM (ii) DDQ (iii) Et3N, BF3OEt2; (b) (i) POCl3/DMF, 80 °C (ii) K2CO3; (c) Amberlyst 15, 80 °C. Inset: optimised structure of 1. | ||
For the spectra see Fig. S1–S4.†
Results and discussion
Synthesis and characterisation
The new BODIPY compound 1 (Scheme 1) was synthesized by following the methods established for such derivatives.40 The structures of 1 as well as intermediates were established using spectroscopic data.A peak at m/z 554.2347 in the high resolution mass spectrum of 1 corresponded to the molecular formula C35H29N4BF2. In the 1H NMR (500 MHz, CDCl3) spectrum, two singlets (3H and 6H, respectively) at δ 2.42 and 3.69 corresponded to ArCH3 and the two indol-1-yl methyl groups. The meso-CH appeared at δ 5.80 as a 1H singlet. While the C-1H of the BODIPY unit appeared as a 1H singlet at δ 6.82 ppm, the C-3 and C-5 Hs were observed at δ 7.82 (s, 2H). A doublet of doublet (J = 2 Hz and J = 4 Hz) at δ 6.47 was assigned to C-6H, while a 1H doublet at δ 6.86 (J = 4 Hz) corresponded to C-7H of the BODIPY unit. The protons of the C-8 aryl ring appeared as two 2H doublets (J = 5 Hz). On the other hand, the indol-2-yl Hs appeared as a 2H singlet at δ 6.65 ppm. The two sets of the C-5′ and 6′ Hs of the two indole rings appeared as two (2H) doublets of triplets (J = 8 Hz, J = 1 Hz) at δ 7.02 and δ 7.20 ppm, respectively. Similarly, the two sets of the C-4′ and 7′ Hs of the two indole rings appeared as two (2H) doublets (J = 8 Hz) at δ 7.24 and δ 7.28 ppm, respectively. The 13C NMR spectrum and heteronucleous single quantum coherence (HSQC) spectrum corroborated the structure of 1 and clearly established 2D heteronuclear chemical shift correlations (see ESI Fig. S5†). Before evaluating the behaviour of 1 towards various analytes, pH titrations were performed, which revealed the stability of 1over a wide pH range (see ESI Fig. S6†), suggesting the pliability of compound 1 for detection of the analyte in environmental and biological settings without resorting to buffered medium. The solution of 1 (5 × 10−6 M, in CH3CN) was non-emissive (ϕf = 0.00541), which is attributed to the photoinduced electron transfer (PET) process.41 The PET mechanism is favoured when the molecular group attached to the fluorophore has the energies of its highest occupied molecular orbital, HOMO (H) and the lowest unoccupied molecular orbital, LUMO (L) levels between the H and L levels of the fluorophore. Thus, simultaneous exchange of electrons from L of the fluorophore to the L of the attached molecular group and H of the latter to H of the fluorophore can occur. Consequently, due to this PET process, the fluorophore would shift to its ground state via a non-radiative process41 thus leading to emission quenching. In order to lend support to our claim, we performed the time dependent density functional theory (TD-DFT) calculations using Gaussian 09 suite of programs.21 As depicted in Fig. 2, on promotion of the electron from the H−7 to L+3 in the BODIPY unit (step i), the higher energy (−5.78 eV) H−2 of bis(1-methyl-1H-indol-3-yl)methyl unit could transfer its electron density to the H−7 (−6.82 eV) of the BODIPY unit (step ii) followed by the electron transfer from L+3 (−0.12 eV) of BODIPY unit to L+1 (−0.74 eV) of bis(1-methyl-1H-indol-3-yl)methyl unit (step iii) and subsequently to H−2 (−5.91 eV) (step iv) causing quenching of the fluorescence emission. Our preliminary investigations revealed that the emission intensity of 1 was significantly enhanced upon addition of aqueous solution of Hg2+ ions (added as perchlorate salt) over a number of other ions including alkali and alkaline earth metal ions: Na+, K+, Ag+, Li+, Ca2+, Mg2+, Ba2+; transition metal ions: Cr3+, Mn2+, Fe2+, Fe3+, Ni2+, Cu2+, Zn2+, Pb2+ (added as perchlorate/nitrate salts) and lanthanides: Sm3+, Pr3+, Ce3+, La3+, Gd3+, Nd3+, Tb3+ (added as their nitrate salts) under similar experimental conditions (see ESI Fig. S7†). Prompted by this observation, we performed elaborate titration experiments in order to quantify the results with Hg2+ ions. The successive addition of an aqueous solution of Hg2+ ions (2.87 × 10−7 M to 3.33 × 10−5 M) to the solution of 1 (5 × 10−6 M, in CH3CN) resulted in a steady increase in emission intensity (ϕf = 0.0682) as well as visually detectable change in solution fluorescence, which attained saturation when addition of 3 × 10−5 M (corresponding to 6.6 equiv.) was complete (Fig. 3). However, saturation was achieved within 2 min when an aqueous solution of Hg2+ corresponding to 6.6 equiv. was instantly added (see ESI Fig. S8†). We attribute this emission enhancement to the interaction of π-electron density of the electron-rich indole groups with that of the acidic Hg2+ ions,42,43 restricting the PET process responsible for the radiation less decay (steps i–iv, Fig. 2). Moreover, the appropriate H orbital of the bis(1-methyl-1H-indol-3-yl)methyl unit gets lowered in energy on complexation with mercury in comparison to free 1, thus inhibiting the electron transfer (step v) to BODIPY core (Fig. 2). The detection limit calculated from the fluorescence data comes out to be 3.3 × 10−7 M which is comparable to most of the probes reported in literature (see ESI Fig. S9 and Table S1†).
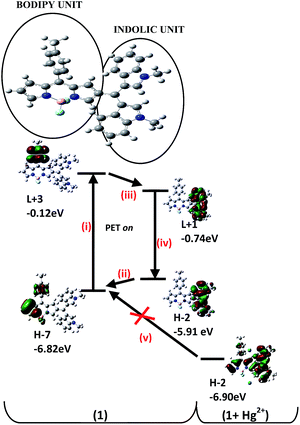 | ||
| Fig. 2 Comparative energies of frontier orbitals of BODIPY and bis(1-methyl-1H-indol-3-yl)methyl unit in the (free and complexed states. H = HOMO, L = LUMO). | ||
Further support of this explanation was accrued from electrochemical studies. Upon gradual addition of aqueous solution of Hg2+ (2 × 10−6 to 3 × 10−5 M) to the solution of 1 (1 × 10−4 M, in CH3CN), the reversible reduction wave at −0.729 V (corresponding to the BODIPY core)44 undergoes anodic shift to −0.643 V (Fig. 4), indicating the depleted electron density on the BODIPY core. Further the competitive experiments performed in the presence of other metal ions, did not affect the emission changes, thereby demonstrating the specificity of 1 for Hg2+ ions (see ESI Fig. S10†). The electronic absorption spectrum of 1 (5 × 10−6 M, in CH3CN) is characterized by two major bands: a weak band centred at 366 nm (ε = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and a strong band 520 nm (ε = 39
000 M−1 cm−1) and a strong band 520 nm (ε = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1), and are assigned as the intramolecular charge transfer (ICT) bands on the basis of TD-DFT calculations, having main contributions from H−1 → L and H−2 → L, H−3 → L, respectively. However, upon gradual addition of aqueous solution of Hg2+ ions (2.87 × 10−7 M to 3.33 × 10−5 M), the absorption spectrum depicted a bathochromic shift (Fig. 5), from 520 nm to 570 nm attaining saturation at 2.87 × 10−5 M addition. The attendant hyperchromic shift of the band at 570 nm caused visual change in the color intensity of the solution. This band at 570 nm has major contributions from the low energy H−2 → L, H−3 → L transitions (ΔE = 2.61 and 2.89 eV, respectively) in comparison to the free 1 (ΔE = 3.23 and 3.76 eV, respectively) (Fig. 6). Fitting the UV-visible titration data using HypSpec, a non-linear least squares fitting programme,45 the binding constant, log
200 M−1 cm−1), and are assigned as the intramolecular charge transfer (ICT) bands on the basis of TD-DFT calculations, having main contributions from H−1 → L and H−2 → L, H−3 → L, respectively. However, upon gradual addition of aqueous solution of Hg2+ ions (2.87 × 10−7 M to 3.33 × 10−5 M), the absorption spectrum depicted a bathochromic shift (Fig. 5), from 520 nm to 570 nm attaining saturation at 2.87 × 10−5 M addition. The attendant hyperchromic shift of the band at 570 nm caused visual change in the color intensity of the solution. This band at 570 nm has major contributions from the low energy H−2 → L, H−3 → L transitions (ΔE = 2.61 and 2.89 eV, respectively) in comparison to the free 1 (ΔE = 3.23 and 3.76 eV, respectively) (Fig. 6). Fitting the UV-visible titration data using HypSpec, a non-linear least squares fitting programme,45 the binding constant, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β1
β1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 3.27 was obtained. The predicted 1
2 = 3.27 was obtained. The predicted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry (1
2 stoichiometry (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Hg2+)2) was further confirmed by the Job plot experiment (Fig. 5, inset). In support of the observed stoichiometry of the possible complex formed through the interaction of 1 and Hg2+, on the basis of DFT calculations, we propose that both the indole groups interact independently with the Hg2+ ions, as shown in the best optimised structure of the putative Hg2+ complex (see ESI Fig. S11†). The energy minimized structure of 1 predicts that benzene ring of one of the indole rings engages in a η4-coordination43 to the Hg(1) with the Hg⋯Cind distances in the range of 2.445 to 3.868 Å and are within the sum of the van der Waals radius of mercury (1.73–2.00 Å)46–48 and that of carbon in heterocyclic aromatic compounds (1.7 Å). However, the benzene ring of the other indole ring is engaged in η2-coordination to the Hg(2) with the Hg⋯Cind distances 3.681 and 3.778 Å. The longer Hg(2)⋯Cind distances in comparison to Hg(1)⋯Cind distances and the different coordination behaviours of Hg2+ ions can be understood as: Hg(2) is also engaged in Hg⋯F interactions (weak) with Hg(2)⋯F distances 3.403 and 3.615 Å within the sum of van der Waals radii 1.30 to 1.38 Å and 1.73 to 2.00 Å of fluorine48 and Hg, respectively.
(Hg2+)2) was further confirmed by the Job plot experiment (Fig. 5, inset). In support of the observed stoichiometry of the possible complex formed through the interaction of 1 and Hg2+, on the basis of DFT calculations, we propose that both the indole groups interact independently with the Hg2+ ions, as shown in the best optimised structure of the putative Hg2+ complex (see ESI Fig. S11†). The energy minimized structure of 1 predicts that benzene ring of one of the indole rings engages in a η4-coordination43 to the Hg(1) with the Hg⋯Cind distances in the range of 2.445 to 3.868 Å and are within the sum of the van der Waals radius of mercury (1.73–2.00 Å)46–48 and that of carbon in heterocyclic aromatic compounds (1.7 Å). However, the benzene ring of the other indole ring is engaged in η2-coordination to the Hg(2) with the Hg⋯Cind distances 3.681 and 3.778 Å. The longer Hg(2)⋯Cind distances in comparison to Hg(1)⋯Cind distances and the different coordination behaviours of Hg2+ ions can be understood as: Hg(2) is also engaged in Hg⋯F interactions (weak) with Hg(2)⋯F distances 3.403 and 3.615 Å within the sum of van der Waals radii 1.30 to 1.38 Å and 1.73 to 2.00 Å of fluorine48 and Hg, respectively.
 | ||
| Fig. 4 Changes in the cyclic voltammogram of 1 (1 × 10−4 M, in CH3CN) upon addition of Hg2+ solution (2 × 10−6 to 3 × 10−5 M, in H2O). | ||
 | ||
| Fig. 5 Absorption spectral changes of 1 (5 × 10−6 M, in CH3CN) upon addition of Hg2+ (2.87 × 10−7 M to 3.33 × 10−5 M, in H2O). Inset: Job's plot. | ||
 | ||
| Fig. 6 Relative energies of the frontier molecular orbitals participating in the low energy electronic transitions of 1 and its complex with Hg2+. | ||
In support of the predicted mercury–fluorine interactions, we recorded the changes in the 19F NMR spectrum of 1 upon adding Hg2+ ion solution (Fig. 7). The spectrum of 1 (δ −142.54, q, J = 30 Hz) showed a small upfield shift (Δδ = 0.35) in the fluorine signals, also accompanied with insignificant change in the coupling constant (ΔJ = 3 Hz) indicating weak interactions between the mercury and fluorine.
The interaction of Hg2+ with 1 was also monitored by 1H NMR titration experiment. For that, titration of 1 was performed by adding increasing concentration of Hg(ClO4)2 (Fig. 8) to a solution (0.6 mL) of 1 (6.0 mM) in CD3CN. A clear shift in the N–Me-indole signals (Δδ = 0.375 ppm) was observed, although the peak positions of p-Me-C6H4 (Δδ = 0.123 ppm) and/or the intensity of other protons was not significantly changed up to the addition of 0.68 equiv. (saturation point) of Hg(ClO4)2. However, upon adding excess amount of Hg(ClO4)2 the observed spectral changes (peak broadening and/or splitting) may be attributed to the heavy atom effect of Hg2+ ions.
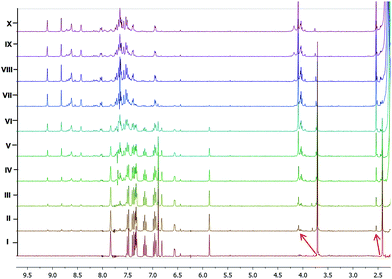 | ||
| Fig. 8 1H NMR titration of 1 (6 mM) (I) with 0.13 (II), 0.26 (III), 0.41 (IV), 0.55 (V), 0.68 (VI), 0.83 (VII), 0.96 (VIII), 1.1 (IX) and 1.25 (X), equiv. Hg(ClO4)2 in CD3CN. | ||
Since reversibility of the sensing protocol is a prerequisite for a molecular probe to be an efficient chemosensor for practical applications, we also studied the reversibility of the sensing event. Thus, the addition of an aqueous solution of cysteine to the in situ formed complex of 1 and Hg2+ leads to the restoration of the original non-emissive behaviour of 1 (see ESI Fig. S12†). The potential application of 1 for the detection of Hg2+ was further confirmed at cellular level using HeLa cells (human cervix carcinoma) as an in vitro model system. Fig. 9 shows the fluorescence images of HeLa cells treated with different concentrations of Hg2+ ions (0, 20 and 30 μM) and 1 (5 μM). The overlay of fluorescent and bright field images revealed the perinuclear fluorescence in cells and thereby depicting the excellent membrane permeability and subcellular localization.
Conclusions
In summary, we have synthesised a new BODIPY derivative which takes advantage of an electron rich bis(1-methyl-1H-indol-3-yl)methyl unit, appended at β-pyrrolic position, in detecting Hg2+ ions in solution as well as live cells. The detection protocol is manifested in the form of ‘turn-on’ emission for which we propose the inhibition of PET in the presence of Hg2+ ions prevailing in otherwise non-fluorescent BODIPY derivative.Acknowledgements
We thank SERB-DST, New Delhi (project SB/S1/OC-45/2013) for financial assistance. NK thanks DST, New Delhi for INSPIRE fellowship and GNDU (UPE) for facilities.Notes and references
- O. A. Bozdemir, R. Guliyev, O. Buyukcakir, S. Selcuk, S. Kolemen, G. Gulseren, T. Nalbantoglu, H. Boyaci and E. U. Akkaya, J. Am. Chem. Soc., 2010, 132, 8029–8036 CrossRef CAS PubMed.
- X. Yin, Y. Li, Y. Zhu, X. Jing, Y. Li and D. Zhu, Dalton Trans., 2010, 39, 9929–9935 RSC.
- M. E. El-Khouly, S. Fukuzumi and F. D'Souza, ChemPhysChem, 2014, 1530–1547 Search PubMed.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- M. T. Whited, P. I. Djurovich, S. T. Roberts, A. C. Durrell, C. W. Schlenker, S. E. Bradforth and M. E. Thompson, J. Am. Chem. Soc., 2011, 133, 88–96 CrossRef CAS PubMed.
- J. IehI, J.-F. Nierengarten, A. Harriman, T. Bura and R. Ziessel, J. Am. Chem. Soc., 2012, 13, 4988–4998 Search PubMed.
- P.-A. Bouit, K. Kamada, P. Feneyrou, G. Berginc, L. Toupet, O. Maury and C. Andraud, Adv. Mater., 2009, 21, 1151–1154 CrossRef CAS.
- B. Brizet, V. Goncalves, C. Bernhard, P. D. Harvey, F. Denat and C. Goze, Chem.–Eur. J., 2014, 20, 12933–12944 CrossRef CAS PubMed.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- R. Misra, T. Jadhav, B. Dhokale, P. Gautam, R. Sharma, R. Maragani and S. M. Mobin, Dalton Trans., 2014, 43, 13076–13086 RSC.
- R. Misra, B. Dhokale, T. Jadhav and S. M. Mobin, Dalton Trans., 2014, 43, 4854–4861 RSC.
- T. K. Khan, R. R. S. Pissurlenkar, M. S. Shaikh and M. Ravikanth, J. Organomet. Chem., 2012, 697, 65–73 CrossRef CAS.
- L. Jiao, W. Pang, J. Zhou, Y. Wei, X. Mu, G. Bai and E. Hao, J. Org. Chem., 2011, 76, 9988–9996 CrossRef CAS PubMed.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- J. Chen, M. Mizumura, H. Shinokubo and A. Osuka, Chem.–Eur. J., 2009, 15, 5942–5949 CrossRef CAS PubMed.
- C. Yu, Y. Xu, L. Jiao, J. Zhou, Z. Wang and E. Hao, Chem.–Eur. J., 2012, 18, 6437–6442 CrossRef CAS PubMed.
- P. Kaur, N. Kaur, M. Kaur, V. Dhuna, J. Singh and K. Singh, RSC Adv., 2014, 4, 16104–21608 RSC.
- N. Kaur, P. Kaur and K. Singh, Sens. Actuators, B, 2016, 229, 499–505 CrossRef CAS.
- B. Dhokale, P. Gautam, S. M. Mobin and R. Misra, Dalton Trans., 2013, 42, 1512–1518 RSC.
- M. J. Frisch, et al., Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford, CT, 2010, for complete reference see ESI ref. 2.
- D. A. Dougherty and J. C. Ma, Chem. Rev., 1997, 97, 1303–1324 CrossRef.
- M. Vedamalai, D. Kedaria, R. Vasita, S. Moric and I. Gupta, Dalton Trans., 2016, 45, 2700–2708 RSC.
- C. Cantürk, M. Üçüncü and M. Emrullahoğlu, RSC Adv., 2015, 5, 30522–30525 RSC.
- E. Karakuş, M. Üçüncü and M. Emrullahoğlu, Chem. Commun., 2014, 50, 1119–1121 RSC.
- S. Madhu, S. Josimuddin and M. Ravikanth, New J. Chem., 2014, 38, 3770–3776 RSC.
- W. Ma, C. Hao, W. Ma, C. Xing, W. Yan, H. Kuang, L. Wang and C. Xu, Chem. Commun., 2011, 47, 12503–12505 RSC.
- Y. Zhu, L. Xu, W. Ma, Z. Xu, H. Kuang, L. Wang and C. Xu, Chem. Commun., 2012, 48, 11889–11891 RSC.
- W. Ma, M. Sun, L. Xu, L. Wang, H. Kuang and C. Xu, Chem. Commun., 2013, 49, 4989–4991 RSC.
- L. Xu, H. Yin, W. Ma, H. Kuang, L. Wang and C. Xu, Biosens. Bioelectron., 2015, 67, 472–476 CrossRef CAS PubMed.
- X. Wu, L. Tang, W. Ma, L. Xu, L. Liu, H. Kuang and C. Xu, RSC Adv., 2015, 5, 81802–81807 RSC.
- W. F. Fitzgerald, C. H. Lamborg and C. R. Hammerschmidt, Chem. Rev., 2007, 107, 641–662 CrossRef CAS PubMed.
- M. E. Pichichero and J. Treanor, Lancet, 2003, 361, 698–699 CrossRef.
- Mercury Update: Impact on Fish Advisories, EPA Fact Sheet EPA-823-F-01-011, EPA Office of Water, Washington, DC, 2001.6.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS PubMed.
- M. Harada, Crit. Rev. Toxicol., 1995, 25, 1–24 CrossRef CAS PubMed.
- H. H. Harris, I. J. Pickering and G. N. George, Science, 2003, 301, 1203 CrossRef CAS PubMed.
- J. C. Clifton, Pediatr. Clin. North Am., 2007, 54, 237–269 CrossRef PubMed.
- E. G. Pacyna, J. M. Pacyana, F. Steenhuisen and S. Wilson, Atmos. Environ., 2006, 40, 4048–4063 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, Berlin, 3rd edn, 2006 Search PubMed.
- P. Kaur, S. Kaur, K. Singh, P. R. Sharma and T. Kaur, Dalton Trans., 2011, 40, 10818–10821 RSC.
- R. Martinez-Manez and F. Sancenon, Chem. Rev., 2003, 103, 4419–4476 CrossRef CAS PubMed.
- D. A. Dougherty, Science, 1996, 271, 163–168 CAS.
- M. R. Haneline, M. Tsunoda and F. P. Gabbai, J. Am. Chem. Soc., 2002, 124, 3737–3742 CrossRef CAS PubMed.
- M. R. Rao, K. V. P. Kumar and M. Ravikanth, J. Organomet. Chem., 2010, 695, 863–869 CrossRef CAS.
- P. Gans, A. Sabatini and A. Vacca, Talanta, 1996, 43, 1739–1753 CrossRef CAS PubMed.
- P. Pyykko and M. Straka, Phys. Chem. Chem. Phys., 2000, 2, 2489–2493 RSC.
- J. Caillet and P. Claverie, Acta Crystallogr., 1975, A31, 448–461 CrossRef CAS.
- A. J. Canty and G. B. Deacon, Inorg. Chim. Acta, 1980, 45, L225–L227 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C NMR, IR spectral data, optimised structures and their Cartesian coordinates, complete ref. 21. See DOI: 10.1039/c6ra17695j |
| This journal is © The Royal Society of Chemistry 2016 |




