Solvothermal fabrication of thin Ag nanowires assisted with AAO
Mingxia Song*a,
Xin Heb,
Chaozhi Zhanga,
Mindong Chena,
Caijin Huangc,
Fenghua Chenad and
Hui Qiua
aJiangsu Collaborative Innovation Center of Atmospheric Environment and Equipment Technology (CICAEET), School of Environmental Science and Engineering, Nanjing University of Information Science & Technology, Nanjing 210044, P. R. China. E-mail: smx839@163.com
bSchool of Applied Physics and Materials, Wuyi University, Jiangmen 529020, Guangdong Province, P. R. China
cState Key Laboratory of Photocatalysis on Energy and Environment, College of Chemistry, Fuzhou University, Fuzhou 350002, China
dHenan Provincial Key Laboratory of Surface and Interface Science, Zhengzhou University of Light Industry, Zhengzhou, P. R. China
First published on 24th August 2016
Abstract
Single crystalline thin silver nanowires with a diameter of ∼45 nm were synthesized using a solvothermal method assisted with an anodic aluminum oxide (AAO) template. AAO here is not playing a role as a hard template but as a heterogeneous medium whose porous structure can promote PVP molecules to form into a one dimensional template. This soft template can effectively guide the growth of Ag nanowires and thus reduce the reaction time considerably. We further investigated the influences of reaction time, temperature and ratio of PVP to AgNO3 on the formation of Ag nanowires by SEM and UV-Vis absorption spectra characterization. The reaction mechanism was also demonstrated by TEM analysis. Our work may provide a better way to control the synthesis of metallic nanostructures in chemical solution.
Introduction
In the last few decades, the synthesis of metallic nanostructures has made tremendous progress driven by their wide applications in optics,1,2 electronics,3 catalysis4–6 as well as biological nanosensors.7,8 From the initial metallic nanostructures such as nanowires,9 nanoplates10 to the recent emerging nanocrystals11,12 or nanoclusters,13,14 the diversity of metallic structures creates more property variations for application explorations.12 The engineering and control of these structures as well as their geometric parameters promote the rapid development of the preparation technology of nanomaterials.Among various metallic nanostructures, one dimensional metallic nanowire is the most common and most studied. Especially, in recent years, the urgent need for one dimensional noble metallic nanowires of some emerging technologies such as plamonics components15–18 and transparent conductive materials19–23 has attracted new interests in the fabrication of noble metallic nanowires. As a common noble metal, Ag has been attracting more interest because of their intriguing electrical, thermal, optical properties and strong surface plasmon resonances (SPRs).9
At present, the methods used to prepare nanowires are usually template method, including hard template24–26 and soft template method,27 seed-mediated method,28–30 hydrothermal/solvothermal synthesis,31–33 microwave-assisted method34 and photochemical/electrochemical reduction method.35,36 Among all methods, solvothermal synthesis is the most widely used. In order to gain the desired nanowires, usually, some surface active agents or traces of salt32,33,37,38 are added to change the morphology of nanostructures or reduce the reaction time. Chen et al. successfully synthesized thin nanowires with diameter of ∼80 nm using solvothermal method by adding traces of CuCl. However the added amount of CuCl has great influence on the formation of Ag nanowires, therefore should be strictly controlled. Meanwhile, the combination of chlorine ion with silver ions partly introduced AgCl impurities.
Herein, we report a very interesting finding of synthesizing thin Ag nanowires using solvothermal method assisted with anodic aluminum oxide (AAO) template. AAO is usually used as a hard template to synthesize aligned nanowires. However, in our work, we demonstrated that AAO played a role of heterogeneous medium which can cause the local high concentration of PVP because of the rough pore structure. When the concentration of PVP reached its critical micelle concentration (CMC), it then formed a rod-like micelle to guide the Ag nanoparticles to grow into nanowires, then consequently largely reduced the reaction time. The effects of reaction temperature, time and PVP/AgNO3 ratio on the morphology and size of nanowires were also discussed in detail.
Experimental
Ag nanowires synthesis
All chemicals were purchased from commercial sources and were used without further purification. Silver nitrates (AgNO3) was purchased from Shanghai Shi Yi Chemicals Reagent Co. Ltd., N,N-dimethylformamide (DMF) was purchased from Tianjin BoDi Chemicals Co. Ltd. And polyvinylpyrrolidone (PVP K30, (C6H9NO)n) was purchased from Shanghai BOAO Biotech Co. Ltd. Anodic aluminum oxide (AAO, Andisc25 with pore diameter 200 nm) was purchased from Whatman International Ltd.18 g PVP was first dissolved in 75 mL DMF in an autoclave of 100 mL. After completely dissolved, 0.51 g AgNO3 was dropwisely added into the solution. Then a piece of AAO template (about 1 cm × 1 cm) was introduced into the reaction solution. Afterwards, the autoclave was sealed and heated at 150 °C for 6 hours. When the reaction is over, the products were cool down to the room temperature. The AAO template was taken out and washed with deionized water for several times. Since in our work, we aimed to study the role of AAO template played in the reaction, so in the following, the products we used to characterize were obtained from the solution.
Characterization
The morphology and geometric dimension were characterized by scanning electron microscope (SEM, JSM – 5610 LV) and JEM-2010 transmission electron microscopes (TEM) with an accelerating voltage of 200 kV. The lattice structure was further observed with High Resolution Transmission Electron Microscopy (HRTEM, JEOL JEM 2100F, 200 kV). The optical properties of prepared Ag nanowires were observed by using a Japanese UV-Vis spectrophotometer (Shimadzu), modeled UV-1601.Results and discussion
In our experiment, Ag nanowires were synthesized by reduced AgNO3 with DMF using solvothermal method. Therefore, the geometrical morphology was greatly affected by the reaction time, temperature and the ratio of different reactants. Fig. 1 shows the SEM images of the products obtained at different temperature: 90 °C, 120 °C and 150 °C while the other experiment parameters were the same. At 90 °C, the main products are almost triangle and irregular hexagonal silver nanoplates with large size. The edge length is more than 1 μm. As the temperature increases to 120 °C, only a very few short nanorods appear. The number of triangle and irregular hexagonal silver nanoplates largely reduces while a lot of irregular Ag nanoparticles appear. As the temperature goes to 150 °C, relatively higher aspect ratio Ag nanowires can be observed. The length is up to dozen or several tens of microns. Interestingly, the nanoparticles almost distribute around the nanowires. Meanwhile the triangle and irregular hexagonal silver nanoplates are still existed. | ||
| Fig. 1 SEM images of products treated under different temperature: (a) 90 °C, (b) 120 °C and (c) 150 °C. | ||
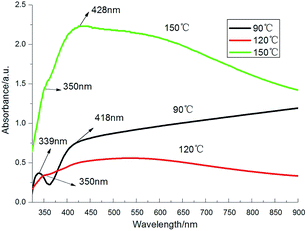
Fig. 2 shows the UV-Vis absorption spectra of the products obtained at different temperature. At about 339 nm, shoulder peak can be seen in all curves. It can be attributed to the out-plane quadrupole resonance of triangle nanoplates while the peaks shown at around 418 nm corresponds to the in-plane quadrupole resonance of triangle nanoplates.39 The shift of adsorption peak may due to the lack of corners and the difference depends on their size and missing degree. The small peak at about 350 nm is close to the characteristics adsorption peak of bulk Ag and also can be attributed to the longitudinal plasmon resonance of nanowires.40,41 The peak at 428 nm indicates the transverse plasmon resonance of nanowires.
The effect of time on the formation of Ag nanowires was also investigated. Fig. 3 shows SEM images of the products treated with various time. In 3 hours, nanowires have already formed which can be seen in Fig. 3(a). The size of all products is small. As the time increases to 6 hours, the nanowires produced increase. Surprisingly, when the reaction time increases to 9 hours, the number of nanowires decreases.
The UV-Vis adsorption spectrum is shown in Fig. 4. Three UV-Vis adsorption curves are similar with each other especially for the curves at 6 h and 9 h. Same as above, the small shoulder peak at about 352 nm indicates the longitudinal plasmon resonance of nanowires. The peak around 428 nm corresponds to the transverse plasmon resonance of nanowires. The peak at 418 nm is the in-plane quadrupole resonance of triangle nanoplates. Since the nanowires and triangle nanoplates co-exist in the products treated with 6 h and 9 h, meanwhile 418 nm is very close to 428 nm, it is not easy to determine the adsorption peak in Fig. 4. The small shift for 6 h and 9 h may due to the relative yield of nanowires and nanoparticles.
The ratio of PVP/AgNO3 used in the experiment is 5, 7 and 9 respectively. Ag nanostructures formed under the three ratios were shown in Fig. 5. When the ratio is 5, the products are almost composed of Ag nanoparticles. There are only few irregular Ag nanoplates and even fewer Ag nanowires produced. When the ratio increases to 7, as we shown above, more nanowires with high aspect ratio formed. As the ratio goes to 9, which indicates more PVP is added, surprisingly, the nanowires totally disappear however more nanoparticles appear. This similar phenomenon was also found by Gao et al.42 In their work, AgNO3 was reduced by ethylene glycol, when the ratio of PVP to Ag+ increased to 4.6, the diameter of nanorods decreased and a lot of nanoparticles occurred. While the molar ratio of PVP/Ag+ increased to 6.5, the yield of nanorods decreased significantly and the major products were equiaxial nanoparticles.42
Since there are no nanowires produced when PVP/AgNO3 = 9, the shoulder peak at 354 nm should be the characteristics adsorption peak of bulk Ag. The peaks at 472 nm and 487 nm in the spectrum for the ratio of 5 and 9 in Fig. 6 suggest the adsorption peaks of irregular spherical nanoparticles.43 The difference of size may cause the shift of the adsorption peak. The peak at 427 nm corresponds to the transverse plasmon resonance of nanowires.
 | ||
| Fig. 6 UV-Vis absorption spectra of the products obtained with different PVP/AgNO3 ratio 5, 7 and 9. | ||
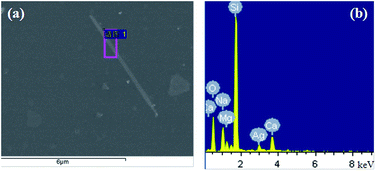
To confirm the element of these structures, a FE-SEM (HITACHIS – 4800) with EDS characterization was used to characterize the product synthesized at 150 °C with reaction time of 6 h and PVP/AgNO3 of 7. We selected the area with only one large nanowire showing in the Fig. 7(a). The EDS spectrum of the selected nanowire marked in the red box suggests that the main elements of this area are Si, Ag, O, Ca, Na and Mg. Among them, Si, O, Ca, Na and Mg are coming from the glass substrate which we used to deposit product while Ag should come from the nanowire structure. Considering the elements percentage in Table 1, it is found that O element is the most because the glass substrate is mainly composed of silica. The percentage of O is almost double of that of Si. Ag is the least due to the only one nanowire in the characterization area.
| Element | wt% | Atom% |
|---|---|---|
| O K | 41.49 | 56.76 |
| Na K | 9.08 | 8.64 |
| Mg K | 2.83 | 2.55 |
| Si K | 35.78 | 27.88 |
| Ca K | 5.74 | 3.14 |
| Ag L | 5.08 | 1.03 |
| Total | 100.00 |
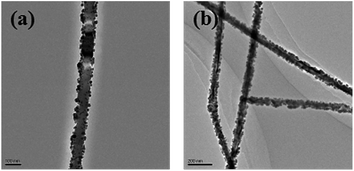
The TEM image in Fig. 8 indicates that the average diameter of these Ag nanowires is about 45 nm and the length can be more than ten micrometers even dozens of micrometers. For nanowires synthesized by polyol method, diameters are usually above 100 nm.44 Chen et al. has successfully synthesized thin nanowires with diameter about 80 nm using a method combining the polyol process and solvothermal with the introduction of cuprous chloride (CuCl).33
From the high-resolution TEM image of single Ag nanowires in Fig. 9, the lattice fringes are clearly seen. The lattice distance is about 2.358 Å which corresponds to the face {111} of the face-centered cubic structure of Ag (2.359 Å, JCPDS: 04-0783). The corresponding FFT transform pattern suggests that there is only one diffraction pattern which indicates the Ag nanowire is single crystalline structure. The diffraction point in the box in Fig. 9(c) corresponds to the lattice distance 1.449 Å indicating the lattice plane {220} of face-centered cubic structure of Ag (1.445 Å, JCPDS: 04-0783).
 | ||
| Fig. 9 (a) High-resolution TEM image of single Ag nanowires (a); (b) magnified image of the area marked with a white box in (a); (c) corresponding FFT transform pattern of box area in (b). | ||
In the whole reaction, DMF is a reducer. The introduction of AAO template plays an important role in the reaction. To investigate the role of AAO template in the reaction, a contrast experiment was performed in the absence of AAO template while the other experimental parameters are the same. The ratio of PVP/AgNO3 is 7 and the reaction temperature is 150 °C. When the reaction time goes to 6 hours, the products are all irregular triangle and hexagon nanoplates that can be seen from the SEM image in Fig. 10(a). When the reaction time increases to 12 hours, there are some Ag nanowires observed in Fig. 10(b). Comparing with the image in Fig. 10(c) with the introduction of AAO template, when the reaction goes to 6 hours, nanowires have already appeared. Obviously, the existence of AAO template can largely reduce the reaction time.
 | ||
| Fig. 10 SEM images of samples treated with different time in the absence of AAO. (a) 6 h; (b) 12 h; and (c) 6 h, with AAO in the reaction solution. | ||
Analyzing the reaction mechanism, in the absence of AAO template, during the early stage of the reaction, Ag seeds grow into silver nanoplates first. Since PVP can play as a crystal capture agent, it interacts more strongly with {110} face than the {111} face.33 It is assumed that as the time increases, the cover of PVP slows down the growth of {110} face. Moreover, the longer reaction time provides more energy to the whole reaction system. In order to lower the overall energy, the sharp corners of Ag nanoplates with high activity tend to dissolve into the solution first, and thus reduce the size of the triangle and hexagonal nanoplates and change its morphology to nanorods. The formed short nanorods then connect with each other and finally grow into nanowires. This may be confirmed by the high resolution TEM images of products obtained in the reaction solution without AAO after 7 hours in Fig. 11. Fig. 11 shows the evolution of Ag nanowires from Ag triangle nanoplates. The Ag triangle nanoplates tend to grow into small nanorods shown in Fig. 11(a) and (b) according to the deduction above. The image in Fig. 11(c) shows the connection of small Ag nanorods.
 | ||
| Fig. 11 High resolution TEM image of nanostructures produced in the reaction solution in the absence of AAO after 7 h. | ||
When AAO is introduced, due to the special rough pore structure, PVP in the solution easily aggregates on the surface of the pore edge or in the vicinity of the template which causes local PVP concentration is higher than that in the other places in the solution. In most literature, PVP is regarded as a polymeric surfactant. Its high concentration can create rod-like micelles. In the suitable ratio range of PVP/AgNO3 (10–1.25),30 when it reaches the critical micelle concentration (CMC), PVP molecules can form a rod micelle structure leading to the formation of one dimensional Ag nanowires.30 The schematic illustration is shown in Fig. 12. The nitrogen and oxygen atoms in the structure of polyvinyl skeleton with polar groups of PVP can complex with Ag through coordination bond, so Ag nanoparticles tend to stick on the rod-like PVP micelle. As more and more nanoparticles stick, they collide and aggregate together and thus the polymer/silver nanocable is formed.30 By centrifugation, the PVP micelle is removed and silver nanowires are obtained. This mechanism can be confirmed by the high resolution TEM images of silver nanowires obtained after 5 hours reaction in Fig. 13. It is clearly seen from Fig. 13, lots of Ag nanoparticles stick on the surface of the rod-like PVP micelle and finally go into the inner surface and then grow into nanowire. Interestingly, AAO here is not playing a role as a hard template however PVP is used as a soft template.
This mechanism also well explained the obvious co-existence of silver nanowires and triangle, hexagonal nanoplates. To improve the yield of nanowires, we may consider placing diffusedly more pieces of AAO templates or prolong the reaction time moderately.
Conclusions
To conclude, we have successfully developed a simple solvothermal method to synthesize thin Ag nanowires using DMF as a reducing agent assisted with AAO template. The reaction temperature, time and the ratio between PVP to AgNO3 were investigated showing their great effects on the morphology and size of Ag nanoparticles. The optimum experimental parameters were thus determined. The reaction mechanism was proposed and the role of AAO template and PVP was emphasized. In this work, AAO template is not playing a role as a hard template but a heterogeneous medium which can cause the local high concentration of PVP. When the concentration reaches to the CMC, the rod-like PVP micelle is formed and guides Ag nanoparticles to grow into one dimensional nanowires. Consequently it largely reduces the reaction time to form Ag nanowires. Since the AAO template is a solid phase in the reaction solution, it is easily separated after the reaction. Instead of adding traces of salts which might introduce impurity ions, our work suggests a better way to control the synthesis of Ag nanowires. It also brings a new idea for the preparation of nanomaterials using wet chemistry method in the future.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant no. 11305091), Nanjing Foundation for Selected Overseas Chinese project, Jiangsu NSF (BK20160946) and Frontier and Key Technological Innovation Special Foundation of Guangdong Province (Grant No. 2014B090915001), and the open project of Jiangsu Engineering Technology Research Center of Environmental Cleaning Materials (No. KFK1506).References
- M. X. Song, A. Bouhelier and G. Colas-des-Francs, et al., ACS Nano, 2011, 5, 5874–5880 CrossRef CAS PubMed.
- J. R. Krenn, B. Lamprecht and F. R. Aussenegg, et al., Europhys. Lett., 2002, 60, 663–669 CrossRef CAS.
- A. I. Hochbaum, R. Chen and P. D. Yang, et al., Nature, 2007, 451, 163–167 CrossRef PubMed.
- A. Canlier, U. V. Ucak and H. Usta, et al., Appl. Surf. Sci., 2015, 30, 79–86 CrossRef.
- M. Han, S. l. Liu and J. Bao, ACS Appl. Mater. Interfaces, 2012, 4, 6654–6660 CAS.
- X. Q. Qiu, Q. W. Liu, M. X. Song and C. J Huang, J. Colloid Interface Sci., 2016, 477, 131–137 CrossRef CAS PubMed.
- J. Homola, S. S. Yee and G. Gauglitz, Sens. Actuators, B, 1999, 54, 3–15 CrossRef CAS.
- G. Barbillon, J. L. Bijeon, J. Plain, M. L. de la Chapelle, P. M. Adam and P. Royer, Gold Bull., 2007, 40(3), 240–244 CrossRef CAS.
- Y. Sun, Nanoscale, 2010, 2, 1626–1642 RSC.
- X. He, X. J. Zhao, Y. Z. Li and X. T. Sui, J. Mater. Res., 2009, 7(24), 2200–2209 CrossRef.
- Y. Yu, Q. B. Zhang, Q. F. Yao, J. P. Xie and J. Y. Lee, Acc. Chem. Res., 2014, 47, 3530–3540 CrossRef CAS PubMed.
- Y. Yu, Q. B. Zhang, J. P. Xie and J. Y. Lee, Nat. Commun., 2013, 4, 1454 CrossRef PubMed.
- Q. F. Yao, X. Yuan, Y. Yu, Y. Yu, J. P. Xie and J. Y. Lee, J. Am. Chem. Soc., 2015, 137, 2128–2136 CrossRef CAS PubMed.
- X. R. Song, N. Goswami, H. H. Yang and J. P. Xie, Analyst, 2016, 141, 3126 RSC.
- M. X. Song, A. Thete, J. Berthelot and A. Bouhelier, et al., Nanotechnology, 2013, 24(9), 95201–95207 CrossRef CAS PubMed.
- W. Wang, Q. Yang, F. Fan, H. Xu and Z. L. Wang, Nano Lett., 2011, 11(4), 1603–1608 CrossRef CAS PubMed.
- C. Rewitz, T. Keitzl, P. Tuchscherer, J. S. Huang, P. Geisler, G. Razinskas, B. Hecht and T. Brixner, Nano Lett., 2012, 12(1), 45–49 CrossRef CAS PubMed.
- P. Kusar, C. Gruber, A. Hohenau and J. R. Krenn, Nano Lett., 2012, 12(2), 661–665 CrossRef CAS PubMed.
- J. Zhu, X. F. Xu, J. L. Liu, Y. Q. Zheng and S. F. Hou, RSC Adv., 2015, 5, 74126–74131 RSC.
- S. H. Park, H. C. Moon and D. H. Lee, RSC Adv., 2016, 6, 50158–50165 RSC.
- T. Kim, A. Canlier and G. K. Kim, et al., ACS Appl. Mater. Interfaces, 2013, 5(3), 788–794 CAS.
- H. W. Ding, Y. J. Zhang and G. B. Yang, et al., RSC Adv., 2016, 6, 8096–8102 RSC.
- X. He, R. H. He, A. Liu and X. Y. Chen, et al., J. Mater. Chem. C, 2014, 2, 9737 RSC.
- R. Yang, C. H. Sui, J. Gong and L. Y. Qu, Mater. Lett., 2007, 61, 900–903 CrossRef CAS.
- P. V. Adhyapak, P. Karandikar, K. Vijayamohanan, A. A. Athawale and A. J. Chandwadkar, Mater. Lett., 2004, 58, 1168–1171 CrossRef CAS.
- Z. A. Hu, T. Xu, R. J. Liu and H. L. Li, Mater. Sci. Eng., A, 2004, 371, 236–240 CrossRef.
- C. Y. Wang, M. H. Chen, G. M. Zhu and Z. G. Lin, J. Colloid Interface Sci., 2001, 243, 362–364 CrossRef CAS.
- F. K. Liu, P. W. Huang, Y. C. Chang, C. J. Ko, F. H. Ko and T. C. Chu, J. Cryst. Growth, 2005, 273, 439–445 CrossRef CAS.
- Y. G. Sun and Y. N. Xia, Adv. Mater., 2002, 14, 833–837 CrossRef CAS.
- K. Zou, X. H. Zhang and X. F. Duan, et al., J. Cryst. Growth, 2004, 273, 285–291 CrossRef CAS.
- T. Y. Zhang, N. Li, M. D. Chen, F. Teng and Y. Hu, Rare Met. Mater. Eng., 2016, 45, 782–787 Search PubMed.
- J. T. Jiu, T. Sugahara, M. Nogi and K. Suganuma, J. Nanopart. Res., 2013, 15, 1588 CrossRef.
- G. Zhu and D. P. Chen, J. Mater. Sci.: Mater. Electron., 2012, 23, 2035–2041 CrossRef CAS.
- Y. Yang, Y. Y. Hu, X. H. Xiong and Y. Z. Qin, RSC Adv., 2013, 3, 8431–8436 RSC.
- W. B. Zhao, J. J. Zhu and H. Y. Chen, J. Cryst. Growth, 2003, 258, 176–180 CrossRef CAS.
- J. J. Zhu, X. H. Liao, X. N. Zhao and H. Y. Chen, Mater. Lett., 2001, 49, 91–95 CrossRef CAS.
- J. J. Ma and M. Zhan, RSC Adv., 2014, 4, 21060–21071 RSC.
- D. P. Chen, X. G. Zhu, G. Zhu, X. L. Qiao and J. G. Chen, J. Mater. Sci.: Mater. Electron., 2012, 23, 625–630 CrossRef CAS.
- R. Jin, Y. Cao and C. A. Mirkin, et al., Science, 2001, 294(30), 1901–1903 CrossRef CAS PubMed.
- Y. G. Sun, Y. D. Yin and B. Mayers, Chem. Mater., 2002, 14(11), 4736–4745 CrossRef CAS.
- Y. Sun, B. Gates, A. B. Mayers and Y. Xia, Nano Lett., 2002, 2(2), 165–168 CrossRef CAS.
- D. L. Chen and L. Gao, J. Cryst. Growth, 2004, 264, 216–222 CrossRef CAS.
- Y. G. Sun and Y. N. Xia, Adv. Mater., 2003, 15(9), 695–699 CrossRef CAS.
- W. Zhang, P. Chen, Q. Gao, Y. Zhang and Y. Tang, Chem. Mater., 2008, 20, 1699–1704 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |