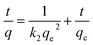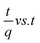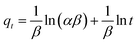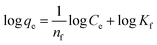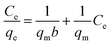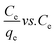Effect of superparamagnetic nanoparticles on the physicochemical properties of nano hydroxyapatite for groundwater treatment: adsorption mechanism of Fe(II) and Mn(II)
Marwa El kady*ab,
Hassan Shokryc and
Hesham Hamad*a
aFabrication Technology Department, Advanced Technology and New Materials Research Institute (ATNMRI), City of Scientific Research and Technology Applications (SRTA-City), New Borg El-Arab City, 21934, Alexandria, Egypt. E-mail: marwa.f.elkady@gmail.com; heshamaterials@hotmail.com; heshamchemistry71185@gmail.com; Fax: +20-2-03-4593414; Tel: +20-2-03-4593414
bChemical and Petrochemical Engineering Department, Egypt-Japan University of Science and Technology (E-JUST), New Borg El-Arab City, Alexandria 21934, Egypt
cElectronic Materials Researches Department, Advanced Technology and New Materials Research Institute (ATNMRI), City of Scientific Research and Technology Applications (SRTA-City), New Borg El-Arab City, P.O. Box 21934, Alexandria, Egypt
First published on 26th August 2016
Abstract
Magnetic hydroxyapatite (MHAP) was found to be an ideal adsorbent for Fe(II) and Mn(II) in ground water from the El-Kharga Oasis in Egypt. The formation of surface iron phases strongly enhances the adsorption capacity of hydroxyapatite-based materials for Fe(II) and Mn(II) ions. Batch sorption studies were implemented to investigate the effect of parameters such as the contact time, dosage of MHAP, pH, agitation speed and temperature on the adsorption process. The results revealed that a good correlation with experimental data was described well by the Langmuir model and the pseudo-second-order model, which explained well the mechanism of adsorption. It was found that the adsorption process was achieved mainly via surface complexation and ion exchange. We found various dominating adsorption mechanisms by changing the initial solution pH. The thermodynamic parameters suggested that the adsorption of Fe(II) and Mn(II) were a non-spontaneous endothermic process. Moreover, the adsorption capacities were affected by several parameters such as contact time, adsorbent dosage, and initial pH. After sorption, the MHAP composites could be effectively and easily separated from aqueous solutions by an external magnet. The results revealed that MHAP had the potential to become a promising material for in situ heavy metal-contaminating groundwater remediation in large scale.
1. Introduction
Groundwater represents one of the important resources for drinking water, and industrial purposes as well as for global food security all over the world.1 Ground water can exist in spaces between loose particles of dirt and rock, or in cracks and crevices in rocks. The Western Desert in Egypt covers approximately 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2, which is more than two-thirds of the total area of Egypt. The significant oases in the Western Desert are Siwa, Bahariya, Farafra, Dakhla and Kharga. The Kharga Oasis is the most southern one in this cluster of depressions and represents an important feature in the Western Desert.2 Ground water is the only water resource for the El-Kharga Oasis. It is obtained from natural freshwater wells and springs fed by the Nubian aquifer. There is a possibility of using both sources deep and shallow ground waters for irrigation. The shallow groundwater in many areas of El-Kharga Oasis has poor quality.3 The costs of drilling deep wells and required energy for extracting groundwater are being very expensive. Therefore, the shallow aquifer in El-Kharga Oasis can be a suitable solution for this problem, where the costs of drilling shallow wells and the required energy for extracting groundwater are very low. Groundwater is generally considered as the best source for potable water as it is well protected from contamination. About 80% of communicable diseases in the world are water-borne. Among the various undesirable and naturally occurring pollutants in water, coli form bacteria, iron and manganese ions, which are very important as they cause severe health problems.4 In the aquifer, groundwater originated from the contact with these solid materials dissolving them, releasing their components, including Fe and Mn into the water.5 They are mostly present in the soluble reduced divalent form as ferrous (Fe(II)) and manganese (Mn(II)) ions. The existence of dissolved iron and/or manganese in groundwater generally infers prior anaerobic conditions with the result that the water is likely to be devoid of oxygen and may also have a high carbon dioxide (CO2) concentration. As well as being associated with groundwater input, the existence of dissolved iron and/or manganese in some deep lakes and reservoirs may be due to stratification, resulting in the development of anaerobic conditions in the bottom water zone and the dissolution of iron and manganese from floor deposits. The dissolved species are subsequently dispersed into the general water body by the annual overturn. Waters containing iron and manganese in solution are clear and colorless. However, on exposure to air or oxygen, such waters become cloudy and turbid due to the oxidation of iron and manganese to the Fe(III) and Mn(IV) states which form colloidal precipitates. The rates of oxidation are not rapid, and thus reduced forms can persist for some time in aerated waters. Concentrations below 0.05–0.1 mg L−1 are usually acceptable to consumers but may sometimes still give rise to the deposition of black deposits in water mains over an extended period; this may vary with local circumstances.6
000 km2, which is more than two-thirds of the total area of Egypt. The significant oases in the Western Desert are Siwa, Bahariya, Farafra, Dakhla and Kharga. The Kharga Oasis is the most southern one in this cluster of depressions and represents an important feature in the Western Desert.2 Ground water is the only water resource for the El-Kharga Oasis. It is obtained from natural freshwater wells and springs fed by the Nubian aquifer. There is a possibility of using both sources deep and shallow ground waters for irrigation. The shallow groundwater in many areas of El-Kharga Oasis has poor quality.3 The costs of drilling deep wells and required energy for extracting groundwater are being very expensive. Therefore, the shallow aquifer in El-Kharga Oasis can be a suitable solution for this problem, where the costs of drilling shallow wells and the required energy for extracting groundwater are very low. Groundwater is generally considered as the best source for potable water as it is well protected from contamination. About 80% of communicable diseases in the world are water-borne. Among the various undesirable and naturally occurring pollutants in water, coli form bacteria, iron and manganese ions, which are very important as they cause severe health problems.4 In the aquifer, groundwater originated from the contact with these solid materials dissolving them, releasing their components, including Fe and Mn into the water.5 They are mostly present in the soluble reduced divalent form as ferrous (Fe(II)) and manganese (Mn(II)) ions. The existence of dissolved iron and/or manganese in groundwater generally infers prior anaerobic conditions with the result that the water is likely to be devoid of oxygen and may also have a high carbon dioxide (CO2) concentration. As well as being associated with groundwater input, the existence of dissolved iron and/or manganese in some deep lakes and reservoirs may be due to stratification, resulting in the development of anaerobic conditions in the bottom water zone and the dissolution of iron and manganese from floor deposits. The dissolved species are subsequently dispersed into the general water body by the annual overturn. Waters containing iron and manganese in solution are clear and colorless. However, on exposure to air or oxygen, such waters become cloudy and turbid due to the oxidation of iron and manganese to the Fe(III) and Mn(IV) states which form colloidal precipitates. The rates of oxidation are not rapid, and thus reduced forms can persist for some time in aerated waters. Concentrations below 0.05–0.1 mg L−1 are usually acceptable to consumers but may sometimes still give rise to the deposition of black deposits in water mains over an extended period; this may vary with local circumstances.6
Dissolved iron and manganese may also form complexes with silica and naturally occurring humic substances. Such complexes may be difficult to oxidize and precipitate and hence to be removed by conventional physicochemical treatment.7,8 Finally, the most common problem limiting the use of groundwater in Egypt is its high iron and/or manganese content. Ferrous (Fe(II)) and manganese (Mn(II)) ions have many environmental and health problems because of their potent toxicity, mutagenicity and carcinogenicity. Long-term exposure to polluted water with high concentration of manganese can damage the respiratory and central nervous system and DNA damage. Also, they can be toxic for the embryo and fetus.9 High concentration of iron can damage blood vessels, because bloody vomitus/stool and damage the liver and kidneys, and even cause death. However, because ingestion is regulated, body tissues are generally not exposed to high-level concentrations. Therefore, it is very important to overcome these problems by treatment before use.10
Heavy metals are serious threat to the environment and public health because of their non-bioaccumulation, non-biodegradable properties, toxicity even at low concentrations and persistent nature.11,12 Iron and manganese are already found in surface water, seawater, groundwater, and drinking-water as formed in complexes or as particulate matter.13 Iron and manganese concentrations in drinking-water often increase during distribution, especially in systems with an acid pH or high-carbonate waters with an alkaline pH.14 The permissible limit of Fe(II) and Mn(II) in water is 0.3 and 0.05 mg L−1, respectively for secondary drinking water according to U.S. Environmental Protection Agency (EPA).9,14
The conventional technologies for the removal of metal ions from polluted water include chemical precipitation,15 ion exchange,16 membrane treatment,17 photocatalysis,18–20 electrochemical treatment and adsorption.21 Chemical precipitation produces large volume of chemical sludge and is highly expensive.22 Hence, researchers have focused on renewable, sustainable, cost-effective, less toxic, highly selective, highly efficient, and simple operational techniques for removal of toxic metal ions from polluted water.23 Adsorption technology is considered as an economic and effective technique for removal of metal ions from ground water.23 The common utilized adsorbents primarily include activated carbons, zeolites, clays, biomass and polymeric materials.23 However, these adsorbent materials suffer from low adsorption capacities and separation inconvenience. Therefore, all efforts are still needed to exploit new investigation to reach for more new advanced adsorbent materials. As the mineral materials have the advantages of wide sources, low cost, simple process, easy use and no regeneration, mineral materials of novel environmental functions will have a great scientific, social and economic significance. It was reported that apatite-group minerals with special crystal chemistry characteristics would become the most promising mineral materials of environmental functions in the treatment processes of polluted water.24 As a member of apatite mineral family, hydroxyapatite (Ca10(PO4)6(OH)2, HAP) is an ideal adsorbent material for decontaminations of polluted ions from water due to its high sorption capacity for metal ions, low water solubility, availability, low cost and high stability under oxidizing and reducing conditions.25 The sorption mechanisms of metal ions from polluted water onto hydroxyapatite based materials are diverse and include mainly the ion exchange, dissolution/precipitation, and formation of surface complexes processes.26 Generally, the small size and high surface area of HAP nanoparticles make these particles especially reactive sorbent materials toward metal ions contaminates from polluted water. However, this small size of HAP nanoparticles as sorbent material had the common drawback of inconvenience to be separated from water treatment media.
Magnetic nanotechnology as a powerful platform for the 21st century technologies could substantially enhance environmental quality and sustainability through pollution prevention, treatment and remediation. The application of this technology to solve environmental problems had received considerable attention in recent years. Where, adsorption process combined with magnetic separation has been used extensively in the water treatment and environmental application.27 The novel invention in this technology is to utilize extra-small super-paramagnetic composite particles, which were conjugated with iron oxide (magnetite) nanoparticles that did not lose their magnetic properties in the presence of a magnetic field. Where, nano magnetite (Fe3O4) has super-paramagnetic features and convenient separation characteristics under a magnetic field.28 Magnetic composites offer advantages due to the easy recovery rapidly, highly efficient and low cost of the adsorbent under an external magnetic field without filtration or centrifugation.29 Magnetic materials combining magnetic separation technology with sorption process have been widely used in environmental pollution cleanup.30 The main advantage of this technology is that it can dispose a mass of wastewater in very short period of time and produces no further contaminants.
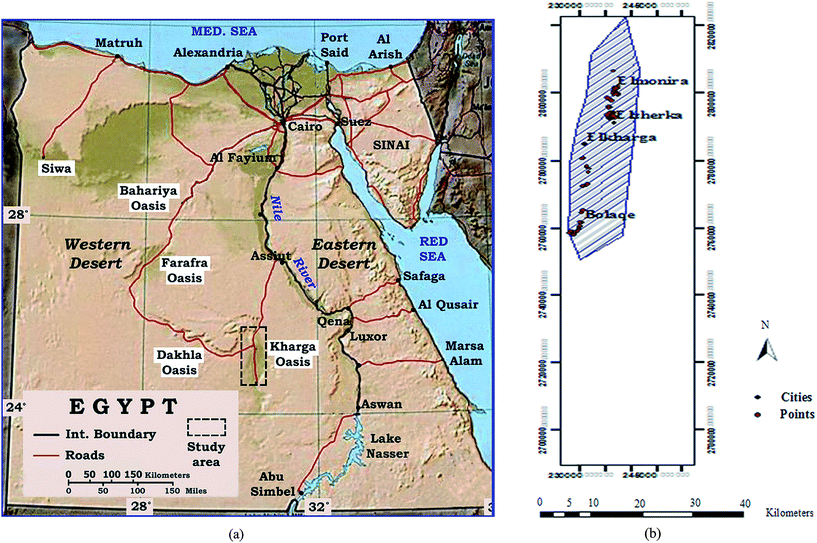
According to the superior functions of nano-magnetite mentioned, the objective of this study is to assess the method for quickly, low energy consumption, low cost and easy handling technique for ground water treatment. This technology will be implemented through developing an efficient advanced adsorbent hydroxyapatite based material which combines in its properties between the advantages of high adsorption capacity of HAP nanoparticles and separation convenience of magnetic materials using microwave irradiation technology. This advanced material will be evaluated as potential adsorbent for manganese and iron ions from ground water collected from El-Kharga Oasis in Egypt using batch mode. So, this work represents the first investigation of utilization magnetic nano-materials for ground water purification especially from iron and manganese ions. The impacts and optimization of adsorbent dosage, temperature, solution pH, contact time and agitation speed on the adsorption process were examined systematically in order to compare the adsorption behaviors of both the prepared HAP and advanced MHAP toward manganese and iron contaminates at El-Kharga Oasis ground water. Additionally, the mechanisms of manganese and ferrous ions adsorption onto the prepared matrices were discussed according to the isotherms, thermodynamics and kinetics models. The HAP and advanced MHAP materials demonstrate remarkable capability toward manganese and iron contaminates at El-Kharga Oasis ground water.
2. Materials and methods
2.1. Ground water sampling
2.2. Batch sorption experiment
In order to determine the optimum adsorption conditions and study the adsorption mechanism of Fe(II) and Mn(II) removal from the polluted ground water using the two synthesized adsorbent materials of hydroxyapatite and magnetic hydroxyapatite, batch adsorption experiment was conducted. All the experiments were performed in triplicate and the average measurements were recorded.Batch adsorption tests were achieved by shaking weighed sample from either the two synthesized adsorbent materials of HAP or MHAP in 50 mL stopper conical flask with 30 mL from El-Kharga Oasis ground water using agitation water bath at 400 rpm for specific time interval. Mn(II) and Fe(II) concentration in ground water before and after treatment process were determined by Inductive Coupling Plasma-Atomic Emission (ICP-AES). The initial concentration (Co) of Mn(II) and Fe(II) ions at El-Kharga Oasis ground water was measured as 50 mg L−1 for each ion. At the end of the adsorption processes, MHAP nano-powder was separated from the treatment solution using permanent magnet. However, HAP nano-powder was separated by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. The percentage of metals removal and the materials adsorption capacity were determined using the following expressions respectively
000 rpm for 15 min. The percentage of metals removal and the materials adsorption capacity were determined using the following expressions respectively
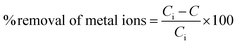 | (1) |
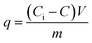 | (2) |
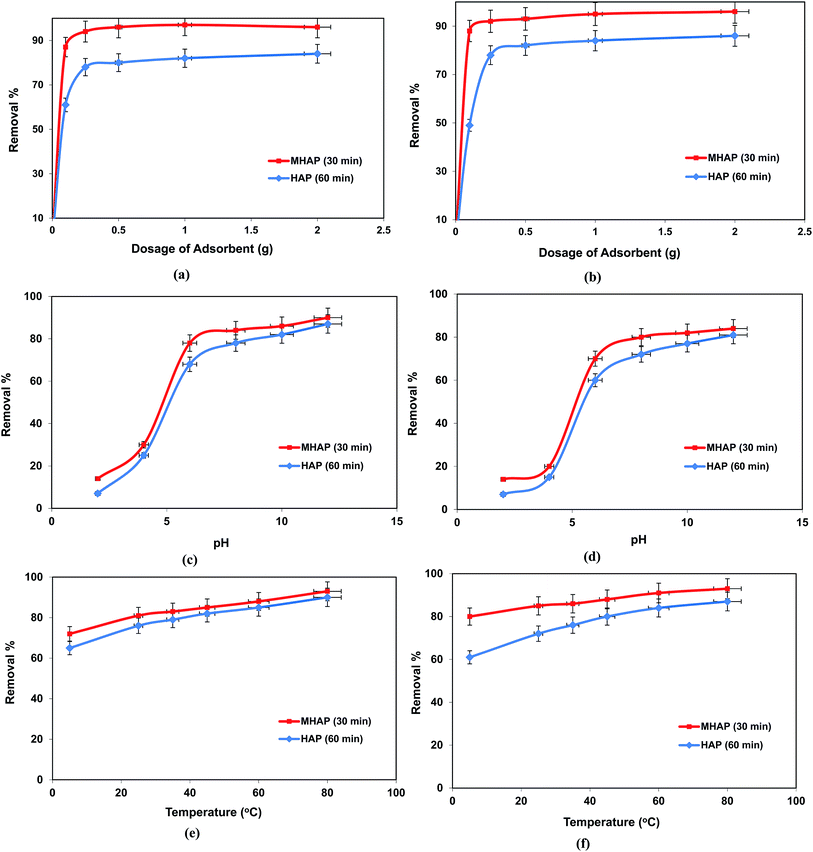
The variation at the different processing parameters through the treatment of El-Kharga Oasis ground water were monitored by examination the effect of contact time over 0–180 min time interval, materials dosage utilized from HAP and MHAP that was changed in the range 0.1–2 g, agitation speed in range 0–400 rpm, solution temperature over 10 °C to 80 °C and the Kharga Oasis ground water pH that was varied in range 1–11. This pH of the ground water solution was adapted using either 0.1 M HCl or 0.1 M NaOH solution according to the required pH value. Adsorption equilibrium for iron and manganese ions adsorption onto the prepared hydroxyapatite based matrices were theoretically modeled using three different models of Langmuir, Freundlich and Temkin equilibrium isotherm models. On the other hand, to describe the adsorption mechanism, the kinetics data were analyzed using five kinetics models of pseudo-first-order, pseudo-second-order, intra-particle diffusion, Elovich and Boyd models. Adsorption thermodynamics of iron and manganese ions onto the prepared matrices were conducted at the studied solution temperatures range.
2.3. Regeneration of MHAP to be reused as an efficient adsorbent material
In order to regenerate MHAP that was contaminated with the adsorbed iron (Fe) and manganese (Mn) ions after the groundwater treatment process, these ions' were desorption using batch experiments. After finishing the treatment period, the contaminated MHAP material was separated from the treatment media by a magnetic field (magnetic bar), and the residual groundwater was analyzed for iron and manganese ions. The metal ions loaded MHAP adsorbent material was washed several times with distilled water and dried at 60 °C. 0.25 g of dried contaminated MHAP was mixed with 50 mL of eluent solutions either 0.05 M Ca(NO3)2 or 0.05 M EDTA and stirred for 180 min at 400 rpm to determine desorption of iron and manganese ions from contaminated MHAP. Liquid samples were withdrawn at predetermined time intervals and analyzed for stripped iron and manganese ions concentrations at the eluents using Inductive Coupling Plasma-Atomic Emission (ICP-AES). All desorption experiments were performed in triplicate and the average measurements were recorded.3. Results and discussion
3.1. Assessment of prepared hydroxyapatite and its magnetic composite for Fe(II) and Mn(II) decontamination from El-Kharga Oasis groundwater
Firstly, mechanism of metal binding onto HAP or MHAP as adsorbent surface can be described by ion exchange mechanism that can be expressed as follows:
Fe(S)2+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca(H)2+ → Ca(H)2+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(H)2+ + Ca(S)2+ Fe(H)2+ + Ca(S)2+
| (3) |
Mn(S)2+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca(H)2+ → Ca(H)2+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn(H)2+ + Ca(S)2+ Mn(H)2+ + Ca(S)2+
| (4) |
The replacement of Ca(II) ions with (Fe(S)2+ or Mn(S)2+) was occurred in two steps. In the first step, at low pH, the presence of metal ions induces the dissolution of HAP to form Ca(II), H2PO4 and water that create partial negative charges onto adsorbent surface. Second step involves exchange of the Ca(II) ions with Fe(II) and Mn(II) ions.34 The alkaline earth metal as Ca(II) is easily replaced by the divalent metal ions due to their exchangeable nature by ion exchange mechanism.35 Therefore, calcium ions presence at the hydroxyapatite based sorbent materials are mainly responsible for metal ions ion exchange process.24 Further increment in the solution pH resulted in decreasing Fe(II) and Mn(II) ions concentration, regarding to the formation of FeOH+ and MnOH+ which decrease Fe(II) or Mn(II) adsorption onto hydroxyapatite based sorbent materials at solution pH higher than 7.
The second suggested mechanism at acidic media is depend mainly on the dissolution and precipitation processes. The higher contents in HAP based material are unstable and they tend to the dissolution–precipitation process that leading to the more stable structure with a higher iron or manganese content. The dissolution–precipitation mechanism can be expressed as follows:
Dissolution
| nCa10(PO4)6(OH)2 + 4mH+ → 10nCa2+ + 3mHPO42− + (2n − m)(PO4)3(OH)10 + mH2O | (5) |
Precipitation
| (x + 5y)M2+ + xmHPO42− + y(PO4)3(OH)10 → xMHPO4 + yM5(PO4)3(OH) | (6) |
The third purposed mechanism is formation of HAP surface complexes, which suggested that at low pH, the medium was destroyed and embedding calcium triangles structure, which induces the protonation at the surface of HAP and MHAP that by its role reduces the number of active sites on the surface of HAP or MHAP.37 On the other hand, at acidic medium, the positively charged hydrogen ions were competing with the Fe(II) and Mn(II) ions at the active sites of the sorbents. It was mentioned that the reactions responsible for the surface properties of HAP in aqueous solutions were:38
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P–O− + H+ ↔ P–O− + H+ ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P–OH P–OH
| (7) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca–OH2+ ↔ Ca–OH2+ ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca–OH + H+ Ca–OH + H+
| (8) |
In the lower pH, the protonation of the surface ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P–O− and
P–O− and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca–OH was occurred and thereby results in increasing the solution pH values. The positively charged
Ca–OH was occurred and thereby results in increasing the solution pH values. The positively charged ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca–OH2+ and neutral
Ca–OH2+ and neutral ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P–OH sites dominated on HAP and MHAP surfaces in acidic solutions, making surface charge of HAP or MHAP in this pH region positive. On the other hand, the final pH values will be decreased in the range of higher initial pH values (5–11) due to hydroxyl anion consumption via deprotonation of surface
P–OH sites dominated on HAP and MHAP surfaces in acidic solutions, making surface charge of HAP or MHAP in this pH region positive. On the other hand, the final pH values will be decreased in the range of higher initial pH values (5–11) due to hydroxyl anion consumption via deprotonation of surface ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca–OH2+ and
Ca–OH2+ and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P–OH sites. Thus, neutral
P–OH sites. Thus, neutral ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca–OH and negatively charged
Ca–OH and negatively charged ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P–O− species predominate in alkaline solutions, causing HAP or MHAP surfaces to become negatively charged in solutions at high pH values.39 Therefore, the sorption of Fe(II) and Mn(II) increases with increasing pH, which can be ascribed to the electrostatic attraction, however, the problem for decomposition of HAP and MHAP via deprotonation still predominated.
P–O− species predominate in alkaline solutions, causing HAP or MHAP surfaces to become negatively charged in solutions at high pH values.39 Therefore, the sorption of Fe(II) and Mn(II) increases with increasing pH, which can be ascribed to the electrostatic attraction, however, the problem for decomposition of HAP and MHAP via deprotonation still predominated.
At neutral pH, the hydroxyl ions present in the HAP and MHAP may not be directly protonated by aqueous protons due to the prevention of the interfacial water molecules from forming hydrogen bonds with the hydroxide ions because of the presence of incorporating calcium triangles in HAP and MHAP crystal structures.
Finally, solution pH = 5 was chosen for further adsorption to avoid the precipitation of Fe(II) and Mn(II) as Fe(OH)2 and Mn(OH)2 at higher pH and the decomposition of adsorbent at an excessive low pH.
| Adsorbent material | Adsorbed polluted metal ions | Adsorbent dosage (g) | Adsorption efficiency (%) | Adsorption time | Adsorption capacity (mg g−1) | References |
|---|---|---|---|---|---|---|
| MHAP | Fe | 2 | 97 | 30 min | 0.704 | Present work |
| MHAP | Mn | 2 | 85 | 30 min | 0.665 | Present work |
| Fe3+ impregnated granular activated carbon (GAC-Fe) | Fe | 8 | 99 | 15 hours | 0.346 | Mondal, P. et al., (2008)48 |
| Fe3+ impregnated granular activated carbon (GAC-Fe) | Mn | 8 | 41 | 15 hours | 0.03 | Mondal, P. et al., (2008)48 |
| Fly ash | Mn | 2 | 28 | 180 min | 0.17 | Mohan, S. and Gandhimathi R. (2009)49 |
| Carbon aerogel | Mn | 10 | 100 | 48 hours | 0.127 | Ajay, K. et al., (2005)50 |
| Lignite | Mn | 0.15 | 100 | 48 hours | Not calculated | Dinesh, M. and Subhash, C. (2006)51 |
| Lignite | Fe | 0.15 | 100 | 48 hours | Not calculated | Dinesh, M. and Subhash, C. (2006)51 |
| Clinoptilolite | Mn | 1 | 65 | 48 hours | 0.7 | Maria, K. (2006)52 |
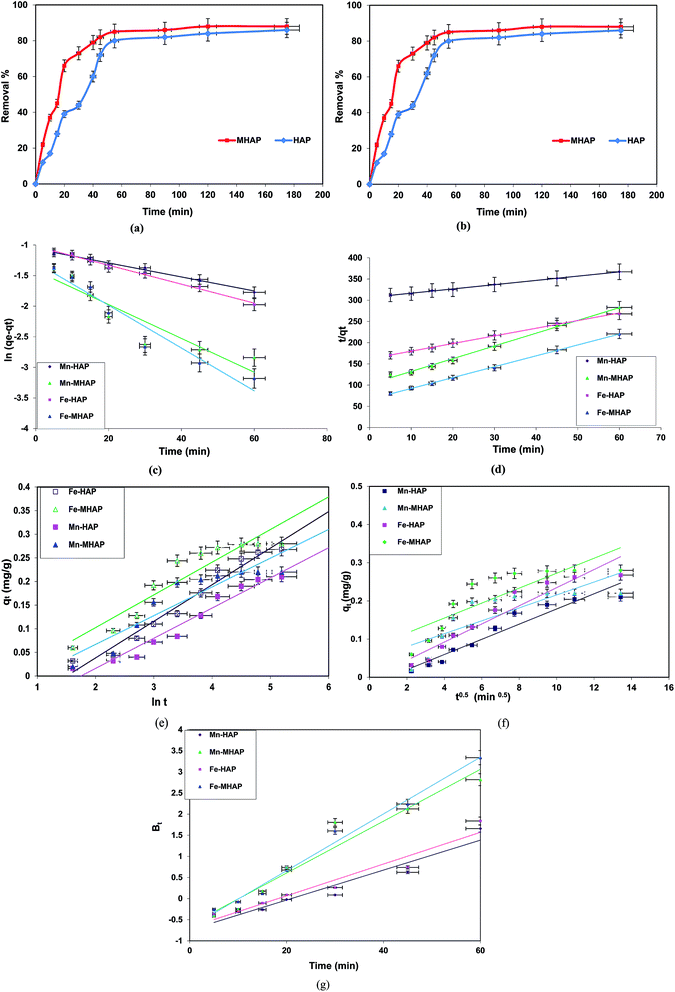
3.1.5.1. Kinetic study and adsorption mechanism. Kinetics study provided important information regarding the mechanism of Fe(II) and Mn(II) sorption onto the prepared HAP and MHAP, which was necessary for determination the efficiency and control the residual time of the total sorption process. The investigation of the adsorption process via a number of kinetic models had been considered as being pseudo-first-order, pseudo-second-order, intraparticle diffusion, Boyd and Elovich equations. These kinetics models are helpful for design of solid/liquid sorption process and the adsorption mechanism by analyzing the rate controlling steps, such as mass transport and chemical reaction processes via five kinetic models. The linear forms of these models (Table 2) were utilized to describe the kinetics of both Fe(II) and Mn(II) onto the prepared HAP or MHAP. The validity of each kinetic model was checked by the fitness of the straight lines of their linear equations as indicated at Fig. 3c–f and their relevant calculative results were summarized at Table 3. As can be seen from this table, the values of the regression coefficients R2 for both pseudo-second order, Elovich and Boyd models were significantly approach to unity compared with other studied models. These results, give prediction that the studied adsorption processes may be controlled by film diffusion or a combination of film adsorption and surface adsorption.
| Kinetic model | Parameter | Mn(II) | Fe(II) | ||
|---|---|---|---|---|---|
| Adsorbent | HAP | MHAP | HAP | MHAP | |
| Pseudo-first-order | k1 (min−1) | 0.0115 | 0.0277 | 0.0155 | 0.035 |
| qe cal. (mg g−1) | 0.168 | 0.212 | 0.224 | 0.272 | |
| qe exp. (mg g−1) | 0.245 | 0.287 | 0.231 | 0.312 | |
| R2 | 0.987 | 0.856 | 0.99 | 0.934 | |
| Pseudo-second-order | k2 (g mg−1 min−1) | 1.0047 | 3.006 | 1.8043 | 2.5623 |
| qe cal. (mg g−1) | 0.168 | 0.212 | 0.224 | 0.272 | |
| qe exp. (mg g−1) | 0.165 | 0.217 | 0.223 | 0.273 | |
| R2 | 0.9974 | 0.9952 | 0.9978 | 0.9993 | |
| Elovich | α (mg g−1 min−1) | 0.0662 | 0.0608 | 0.0774 | 0.0692 |
| β (g mg−1) | 0.1116 | 0.0548 | 0.1165 | 0.0359 | |
| qe cal. (mg g−1) | 0.157 | 0.199 | 0.211 | 0.258 | |
| qe exp. (mg g−1) | 0.165 | 0.217 | 0.223 | 0.273 | |
| R2 | 0.993 | 0.984 | 0.95 | 0.97 | |
| Intraparticle diffusion | I (mg g−1) | 0.0198 | 0.0464 | 0.0028 | 0.077 |
| kint (mg g−1 min−0.5) | 0.0199 | 0.0169 | 0.0237 | 0.0196 | |
| R2 | 0.954 | 0.957 | 0.9616 | 0.96 | |
| Boyd | R2 | 0.9628 | 0.9671 | 0.9553 | 0.9873 |
The pseudo first order assumes that the uptake rate is limited by only one process or mechanism acting on a single class of adsorbing sites.48 It was evident from Table 3 and Fig. 3c that pseudo first order did not provide an accurate calculated adsorption capacities for all prepared materials compared to experimental data. Moreover, the values of R2 are relatively far from the linearity. Thus, this model is not suitable to describe the entire sorption processes onto heterogeneous surfaces since numerous sorption sites and mass transfer may be included at the process mechanism. However, pseudo-second-order kinetic model Fig. 3d recorded the highest satisfactory linear fitting compared to pseudo-first-order model, where, the values of regression coefficients for all prepared materials are higher than 0.99 for the two studied metal ions. Furthermore, the calculated adsorption capacities for all prepared materials are adequate to the experimental capacities for iron and magnesium ions. These results indicate that the sorption processes of both Fe(II) and Mn(II) onto the prepared materials obeyed pseudo-second-order kinetic which confirm that the Fe(II) and Mn(II) ions decontamination process onto prepared HAP based materials is chemisorption process.
It is predicted that the adsorption behavior of both iron and magnesium ions onto the prepared hydroxyapatite based materials is predominated over the whole sorption processes, as the action of both magnetite and HAP as an effective adsorbent materials. However, according to obeying the sorption kinetic processes for pseudo-second-order kinetic model, it can be predict that the overall rate of adsorption of both Fe(II) and Mn(II) over the prepared materials seems to involve valence forces through chemical reaction or exchange of electrons between adsorbent and adsorbate due to the presence of calcium ions at HAP prepared based materials that are responsible for the ion exchange process.49 This prediction was confirmed through the linear fitting of qt versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t for the Elovich kinetic model. Fig. 3e showed that the kinetics of Fe(II) and Mn(II) adsorption processes onto the prepared materials can be explained by Elovich equation. In addition, it was concluded from Table 3 that the values of R2 are greater than 0.95. Also, the calculated qe were identical to experimental qe. These results confirm that the studied sorption processes onto the prepared hydroxyapatite based nano-materials is controlled by chemisorption process. This gives predication that the sorption process of polluted metal ions at El-Kharga Oasis ground water onto HAP and its magnetic composites (MHAP) may involve valence forces through sharing or exchange the electrons between adsorbent and adsorbate.50 This may be corresponding to the exchangeable H+ ions that are present at O–H groups present at hydroxyapatite structure. So; the ion exchange mechanism plays an important role in the sorption processes for treatment El-Kharga Oasis ground water from both iron and magnesium ions.
t for the Elovich kinetic model. Fig. 3e showed that the kinetics of Fe(II) and Mn(II) adsorption processes onto the prepared materials can be explained by Elovich equation. In addition, it was concluded from Table 3 that the values of R2 are greater than 0.95. Also, the calculated qe were identical to experimental qe. These results confirm that the studied sorption processes onto the prepared hydroxyapatite based nano-materials is controlled by chemisorption process. This gives predication that the sorption process of polluted metal ions at El-Kharga Oasis ground water onto HAP and its magnetic composites (MHAP) may involve valence forces through sharing or exchange the electrons between adsorbent and adsorbate.50 This may be corresponding to the exchangeable H+ ions that are present at O–H groups present at hydroxyapatite structure. So; the ion exchange mechanism plays an important role in the sorption processes for treatment El-Kharga Oasis ground water from both iron and magnesium ions.
The existence of other controlling processes affecting the sorption mechanism such as mass transfer or intra-particle diffusion is difficult to be identifying using either pseudo-second order or Elovich kinetic models.
Webber's pore-diffusion model (intraparticle diffusion model) and Boyd's model are the two most widely used models for describing the mechanism of adsorption process. The linear fitting results of the Fe(II) and Mn(II) adsorption processes onto the prepared materials (Fig. 3f) were in conformity with the intraparticle diffusion model. Table 3 evident that the R2 values for this diffusion model were between 0.954 and 0.9616 suggesting that the sorption processes can be followed by an intra-particle diffusion model. These results indicated that the metal ions adsorption processes could be take place through the following four steps: (1) transport of the adsorbate (Fe(II) or Mn(II)) in the bulk solution, (2) film diffusion of these solutes at boundary layer or migration or diffusion of metal ions from the bulk solution to external surface of adsorbent material, (3) transfer of the metal ions into the intraparticular active sites and/or pores (intraparticle diffusion) at the adsorbent material, (4) diffusion through small pores of adsorbent, chemical binding reaction of the adsorbate that is followed by the final equilibrium adsorption and the maximum adsorption was obtained.51–53 Due to the double nature of intraparticle diffusion (both film and pore diffusion) model, and in order to determine the actual rate-controlling step involved in adsorption of both Fe(II) and Mn(II) onto the prepared hydroxyapatite based materials, the kinetic data was further analyzed by Boyd model. This model distinguished the actual slow step in the sorption process for solute transport, whether the process was controlled by the external transport (film diffusion) or intra-particle transport resistance. Fig. 3g indicated that the plot of Bt versus t for Fe(II) and Mn(II) adsorption from the polluted ground water onto the prepared hydroxyapatite based materials were straight lines that do not pass through the origin, indicating that film diffusion governs the rate limiting processes.
Finally, based on the kinetic modeling results, it was demonstrated that adsorption of Fe(II) and Mn(II) from the polluted El-Kharga Oasis ground onto the prepared hydroxyapatite based materials are governed by surface chemisorptions that occurred at the boundary layers of the prepared materials and intra-particle-diffusion mechanisms that controlled by film diffusion.
3.2. Adsorption isotherms
The adsorption isotherms are important mathematical models to find the suitable model that can be used for design purpose. It reflect the surface characteristics of the adsorbent at micro level based on the possibility of the interaction with adsorbate and the homogeneity or heterogeneity of the solid surface and the type of coverage between adsorbent and adsorbate. Adsorption isotherms provide some physicochemical information on how the adsorption occurs and how the reaction between adsorbate and adsorbent surface proceed.54 The adsorption data of HAP and its magnetic composites (MHAP) were analyzed using three important isotherms Freundlich,55 Langmuir56 and Temkin57 equations. The linear forms of these isotherms with their plots are given in Table 4. The linear plotting of these three adsorption models was investigated at Fig. 4. The isotherm parameters calculated from the slope and intercept of the linear plotting were presented at Table 4. Langmuir isotherm is originally developed to represent chemisorptions on a set of distinct, localized adsorption sites.58 Table 5 shows high regression correlation coefficient R2 > 0.96 for Langmuir model. This result indicates that Langmuir model is appropriate for describing the sorption of Fe(II) and Mn(II) onto prepared HAP and MHAP. Table 5 evident that the calculated mono-layer adsorption capacities (qm) for Fe(II) and Mn(II) adsorption onto HAP were 0.539 and 0.6112 mg g−1 respectively. Also, the calculated values of metal ions adsorption capacities onto MHAP were recorded as 0.704 and 0.665 mg g−1 for Fe(II) and Mn(II) respectively. These results indicate that both HAP and MHAP had better adsorption affinity for Mn(II) and Fe(II) compared with other adsorbent materials as evident from Table 1. Subsequently, the strong electrostatic attraction is occurred between Mn(II) ions and the prepared adsorbent materials bending sites. The suitability of the Langmuir adsorption isotherm designated the homogenous and monolayer type of adsorption onto the prepared materials surfaces with finite number of identical adsorption sites with no transmigration of adsorbate in the plane of surface and uniform adsorption energies that confirm the ion exchange mechanism is predominated through this process.59 Also, adsorption process is independent of the neighboring sites occupancy.60 In order to find out the feasibility of the isotherm, the essential characteristics of the Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor or equilibrium parameter, RL
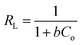 | (9) |
| Correlation parameters | Parameter | Mn(II) | Fe(II) | ||
|---|---|---|---|---|---|
| Adsorbent | HAP | MHAP | HAP | MHAP | |
| Freundlich | Kf (mol1−n Ln g−1) | 1.429 | 2.288 | 1.460 | 2.491 |
| n | 2.161 | 2.637 | 2.602 | 2.471 | |
| R2 | 0.94 | 0.98 | 0.97 | 0.977 | |
| Langmuir | qm (mg g−1) | 0.6112 | 0.704 | 0.539 | 0.665 |
| b (L mg−1) | 0.64 | 0.89 | 1.55 | 1.83 | |
| R2 | 0.963 | 0.999 | 0.995 | 0.99 | |
| RL | 0.913–0.016 | 0.921–0.053 | 0.954–0.021 | 0.915–0.0371 | |
| Temkin | KT (L mg−1) | 11.68 | 9.487 | 9.14 | 78.257 |
| B | 0.138 | 0.2435 | 0.258 | 0.174 | |
| R2 | 0.967 | 0.982 | 0.969 | 0.984 | |
The Freundlich isotherm is an empirical model that applied to non-ideal on heterogeneous surface energies, in which the energy term in the Langmuir equation varies as a function of the surface coverage as well as multilayer adsorption.61 The values of 1/n and Kf for the prepared adsorbents were calculated from the slope and the intercept of the linear plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe vs. log
qe vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce and are listed in Table 5. The values of n giving an indication of how favorable the adsorption process of Fe(II) and Mn(II) ions onto the prepared materials. The slope n ranging between 0 and 1 is a measure of sorption intensity or surface heterogeneity, becoming more heterogeneous as its value gets closer to 0. When 1/n values are in the range 0.1 < 1/n < 1, the adsorption process is desirable.62 Table 5 showed that the calculated Kf values for MHAP were higher than HAP for the two studied metal ions which indicates that the sorption of Fe and Mn is highly affected by incorporating the magnetic materials. This result confirm the vital role of immobilized magnetite nano-particles onto prepared MHAP material as an effecitive adsorbent material. Comparing the regression coefficient values (Table 5) for both Langmuir and Freundlich isotherms, it was demonstrated that the Langmuir isotherm was the most suitable isotherm to describe the equilibrium data for Fe and Mn metal ions sorption.
Ce and are listed in Table 5. The values of n giving an indication of how favorable the adsorption process of Fe(II) and Mn(II) ions onto the prepared materials. The slope n ranging between 0 and 1 is a measure of sorption intensity or surface heterogeneity, becoming more heterogeneous as its value gets closer to 0. When 1/n values are in the range 0.1 < 1/n < 1, the adsorption process is desirable.62 Table 5 showed that the calculated Kf values for MHAP were higher than HAP for the two studied metal ions which indicates that the sorption of Fe and Mn is highly affected by incorporating the magnetic materials. This result confirm the vital role of immobilized magnetite nano-particles onto prepared MHAP material as an effecitive adsorbent material. Comparing the regression coefficient values (Table 5) for both Langmuir and Freundlich isotherms, it was demonstrated that the Langmuir isotherm was the most suitable isotherm to describe the equilibrium data for Fe and Mn metal ions sorption.
Temkin adsorption isotherm model has been developed for considering the liberation of heat of adsorption decreases linearity with coverage due to adsorbate–adsorbent interactions. Furthermore, this isotherm model based on the assumption of that the adsorption was characterized by a uniform distributing the binding energies.63 The values of KT and B were determined from the slope and intercept of the linear plot qe vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce and were listed in Table 5. From Table 5, the correlation coefficients for Temkin isotherm models (>0.95) indicated that the adsorption system based in the heat and the metal ions sorption processes were characterized by a uniform distribution of binding energies, up to some maximum binding energy.
Ce and were listed in Table 5. From Table 5, the correlation coefficients for Temkin isotherm models (>0.95) indicated that the adsorption system based in the heat and the metal ions sorption processes were characterized by a uniform distribution of binding energies, up to some maximum binding energy.
3.3. Adsorption thermodynamic
Temperature plays important role in adsorption process by creating new binding adsorption sites through rupturing of existing bonds and increasing the mobility of metal cations. In order to evaluate the spontaneity and heat change for the adsorption processes and investigate the possible mechanism involved in the adsorption progress. During the thermodynamic study, it was observed that the better adsorption performance of Fe(II) and Mn(II) ions was increased with increasing solution temperature. Thus, the adsorption process was temperature-dependent in nature. The thermodynamic behaviors were evaluated by the following equations:
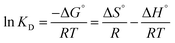 | (10) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD versus 1/T is straight line with an acceptable correlation coefficient (R2). Thermodynamic parameters of free energy change (ΔG°), standard enthalpy changes (ΔH°) and standard entropy change (ΔS°) were calculated by Khan and Singh method.64 The calculated values of ΔH°, ΔS°, and ΔG° were listed in Table 6. The positive values of ΔH° indicate the endothermic nature of adsorption and strong interaction between adsorbent and adsorbates as the action of ion exchange procees onto prepared HAP based material. The explanation for positive entropy is that the hydration sheath of metal ions had to be destroyed before its adsorption on the adsorbent and this dehydration process needs consumes energy.32,39 So, this result confirms that the sorption processes are favored at high temperatures which are confirmed in Fig. 4. The negative values of ΔS° indicate that the adsorption leads to decrease disorder at the solid/liquid interface during adsorption process on adsorbent materials, and is supportive of the interaction between metals ions and adsorbent materials. As the temperature increases, the mobility of metal ions was increased causing the ions to escape from the solid phase to the liquid phase. Therefore, the amount of metals that can be adsorbed will decrease.65 The thermodynamic analysis suggests that the sorption processes of Fe(II) and Mn(II) onto HAP and MHAP area nonspontaneous and have endothermic nature.
KD versus 1/T is straight line with an acceptable correlation coefficient (R2). Thermodynamic parameters of free energy change (ΔG°), standard enthalpy changes (ΔH°) and standard entropy change (ΔS°) were calculated by Khan and Singh method.64 The calculated values of ΔH°, ΔS°, and ΔG° were listed in Table 6. The positive values of ΔH° indicate the endothermic nature of adsorption and strong interaction between adsorbent and adsorbates as the action of ion exchange procees onto prepared HAP based material. The explanation for positive entropy is that the hydration sheath of metal ions had to be destroyed before its adsorption on the adsorbent and this dehydration process needs consumes energy.32,39 So, this result confirms that the sorption processes are favored at high temperatures which are confirmed in Fig. 4. The negative values of ΔS° indicate that the adsorption leads to decrease disorder at the solid/liquid interface during adsorption process on adsorbent materials, and is supportive of the interaction between metals ions and adsorbent materials. As the temperature increases, the mobility of metal ions was increased causing the ions to escape from the solid phase to the liquid phase. Therefore, the amount of metals that can be adsorbed will decrease.65 The thermodynamic analysis suggests that the sorption processes of Fe(II) and Mn(II) onto HAP and MHAP area nonspontaneous and have endothermic nature.
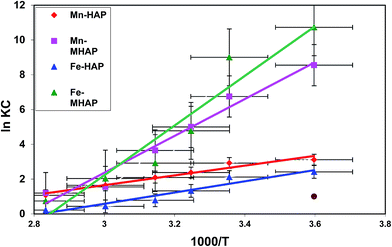 | ||
| Fig. 5 Van't Hoff plot for Fe(II) and Mn(II) adsorption onto HAP and MHAP (Co = 50 mg L−1, V = 50 mL, contact time = 120 min, pH = 5, temperature = 298 K). | ||
| Matrix and ions | Slope (ΔH/R) | ΔH (kJ mol−1) | Intercept (ΔS/R) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1) | R2 |
|---|---|---|---|---|---|---|
| Mn-HAP | 2.812 | 23.39022 | −6.7853 | −56.4401 | 20.45 | 0.955 |
| Mn-MHAP | 10.592 | 88.10426 | −29.409 | −244.624 | 88.52 | 0.9632 |
| Fe-HAP | 3.2315 | 26.87962 | −9.1146 | −75.8152 | 27.43 | 0.9386 |
| Fe-MHAP | 14.276 | 118.7478 | −40.604 | −337.744 | 12.22 | 0.9479 |
3.4. Mechanism discussion
For HAP based sorbent materials, the positively charged calcium ions and negatively charged phosphate groups on HAP surfaces render it useful as a solid matrix for the separation of anion and cation species from aqueous solutions. In spite of the advantages of HAP, all the adsorbents based on HAP nanoparticles had the common drawback of inconvenience to be separated from the treatment media. Therefore, Fe3O4/HAP composite material was substituted to be utilized as magnetic adsorbent material, where Fe3O4 was impregnated onto the surface of HAP for Mn(II) and Fe(II) adsorption. The most prominent advantage of the prepared MHAP as adsorbent with super-paramagnetism and adsorption capacities was its separation convenience from aqueous solutions.To clarify the sorption of Fe(II) and Mn(II) on HAP and MHAP, three possible mechanisms, including electrostatic, ion exchange and surface complexation have been proposed.66
It well known that Ca(II) ions in the HAP could be easily ion exchanged with various other metal ions.67 The ionic radius of Fe(II) (0.065 nm) and Mn(II) (0.075 nm) is smaller than that of Ca(II) (0.099 nm). It is reasonable that Ca(II) could be easily substituted in the HAP crystal lattice. This data support the suggested ion-exchange mechanism of Fe(II) and Mn(II) sorption onto HAP and MHAp (more details about ion exchange mechanism in Section 3.1.2.).
It is noted that after the sorption of Fe(II) and Mn(II) onto HAP and MHAP, the pH values of the treatment solutions decreased. This indicated that the sorption of Fe(II) and Mn(II) results in proton release from the surface ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) POH sites on the HAP based materials in aqueous solution according to the next reactions:
POH sites on the HAP based materials in aqueous solution according to the next reactions:
| HAP-OH + Fe2+ ↔ HAP-O-Fe2+ + H+ | (11) |
| HAP-OH + Mn2+ ↔ HAP-O-Mn2+ + H+ | (12) |
| 2HAP-OH + Fe2+ ↔ (HAP-O)2-Fe + H+ | (13) |
| 2HAP-OH + Mn2+ ↔ (HAP-O)2-Mn + H+ | (14) |
Eqn (11)–(14) suggests the formation of surface complexes onto prepared HAP based materials.66
It is concluded that electrostatic, ion exchange and surface complexation are alternatively dependable for the sorption of Fe(II) and Mn(II) on HAP and MHAP. This might be referred to the various functionalities of the HAP and MHAP. Both the magnetic materials and hydroxyapatite can cooperate to reach the highest efficient removal of Fe(II) and Mn(II) ions from an aqueous solutions.
3.5. Application of MHAP to real ground wastewater treatment
Results stemmed from our pervious optimization by batch mode were applied for the treatment of real ground wastewater using MHAP as adsorbent. The as-prepared MHAP can be effectively used for Fe(II) and Mn(II) removal and presents the following benefits for environmental technology and economy. (i) MHAP showed superior performance and simple feasibility of this process. (ii) Some composites adsorbents perform better for remove one or two pollutants in water, but they have complex materials or an expensive. The cost of 1 kg of MHAP is 250$ including all expenses (materials, transportation, handling, chemicals, electrical energy, drying, etc.). (iii) MHAP can also be easily separated from water by placing an external magnetic field for operation and management of groundwater treatments.In the synthesis of MHAP, the economic cost and environmental application into consideration for the need of the El-Kharga's people for clean fresh water, MHAP has a better prospect in adsorption or other field.
3.6. Regeneration of MHAP to be reused as an efficient adsorbent material
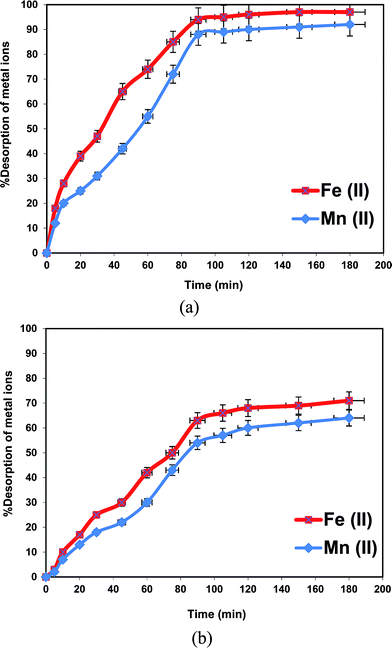
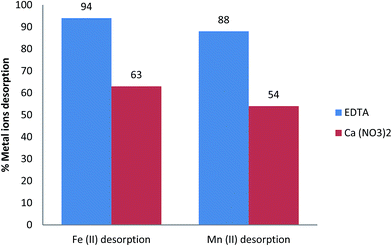
In particular, magnetic-based nano-adsorbents can be produced at relatively low cost. The high adsorption capacity, low cost, easy separation and regeneration make magnetic-based nano-adsorbents technologically and economically advantageous. The main purpose of the recovery and reuse of magnetic-based nanoparticles is to reduce the overall water treatment process costs and energy consumption. The magnetic properties of these materials facilitate their separation from the treatment media using an external magnetic field after finishing the water treatment process without any requirement for power sources, such as the centrifugational force for material separation. So, the contaminated MHAP loaded with both iron and manganese ions were regenerated using either EDTA or calcium nitrate solutions as an eluents to be reused again as an efficient adsorbent material for groundwater treatment. Generally, it was indicated from Fig. 6 that both Fe and Mn desorption significantly increased with increasing desorption time. Furthermore, the amount of desorbed Fe ions is slightly greater than that of Mn ions for the same desorption time. This behavior owed to desorption of bound cations on adsorbent material is depending mainly on both the sorption level and solution composition.68 It was recorded previously that MHAP has greater Fe sorption efficiency compared with Mn efficiency at the same sorption time. Accordingly, the desorption amount of Fe ions was greater than Mn ions. Fig. 7 showed that the percentage desorption of both Fe and Mn ions using EDTA as an eluent solution was increased with desorption time until 90 minutes. After this time the percentage ions desorption almost remains constants. So, the equilibrium time of metal ions desorption from MHAP was recorded as 90 minutes using EDTA as eluent solution. The maximum percentage desorption at the equilibrium desorption time was 94% and 88% for Fe and Mn ions respectively. Unlike, utilizing calcium nitrate as an eluent solution the percentage of metal ions desorption was increased with increasing desorption time above 90 minutes. Moreover, the percentage of Fe and Mn desorption using Ca(NO3)2 was smaller than that using EDTA as eluent solution. Fig. 7 indicated that the same desorption time of 90 min (equilibrium time using EDTA) the percentage Fe ions desorption from MHAP was decreased from 94% using EDTA to 63% using Ca(NO3)2. Furthermore, the percentage Mn ions desorption from MHAP was decreased from 88% using EDTA to 54% using Ca(NO3)2. This phenomenon may be attributed to the formation of complex between EDTA and metal ions which possesses lower sorption affinity for MHAP adsorbents.69 These results confirm the availability of regeneration of MHAP to be reused as an efficient adsorbent material for groundwater treatment.4. Conclusion
The present results strongly indicate that the adsorption potential of the synthesized HAP and Fe3O4/HAP (MHAP) nano-composite materials showed significant removal of both Fe(II) and Mn(II) ions from ground water at El-Kharga Oasis in Egypt. The following conclusions were obtained from the study.(1) Fe3O4 in MHAP showed significant improvement of the adsorption rather than pristine materials. Also, both the prepared HAP and MHAP materials had better affinity for manganese ions rather than iron ions.
(2) Adsorption kinetics data of Fe(II) and Mn(II) sorption onto prepared materials obeyed pseudo-second-order model very well. However, the equilibrium adsorption isotherm was best fitted by Langmuir model. The maximum removal of both metal ions on magnetic composite was pH 4.5. Furthermore, the thermodynamic studies revealed that the adsorption process was non-spontaneous and endothermic in nature.
(3) The Fe(II) and Mn(II) sorption processes from the polluted El-Kharga Oasis ground onto HAP and MHAP were dominated by ion exchange, surface complexation and electrostatic mechanisms. The sorption processes are governed by surface chemisorptions that occurred at the boundary layers of the prepared materials and intra-particle-diffusion mechanisms that controlled by film diffusion.
(4) MHAP would be promising adsorbent for removing iron and manganese ions from groundwater in terms of high efficiency, feasibility, stability, reactivity, regeneration and environmentally friendly, as well as simple and convenient magnetic separation from the treatment media.
Acknowledgements
This work is supported by the key project funded by Science and Technology Development Fund (STDF) (Grant no. 10763).References
- A. Basu, D. Saha, R. Saha, T. Ghosh and B. Saha, Res. Chem. Intermed., 2014, 40, 447 CrossRef CAS.
- A. Salman, F. Howari, M. El-Sankary, A. Wali and M. Saleh, J. Afr. Earth Sci., 2010, 58, 341 CrossRef.
- M. El Tahlawi, A. Farrag and S. Ahmed, Environ. Geol., 2008, 55, 639 CrossRef.
- D. Nemade, M. Kadam and S. Shankar, Ecol. Eng., 2009, 35, 1152 CrossRef.
- R. Abdellatif, M. Ramadan and M. Kamel, Egypt J. Eco. Health Environ., 2016, 4, 21 Search PubMed.
- M. Wong, J.–Am. Water Works Assoc., 1984, 76, 76 Search PubMed.
- T. Hedberg and A. Wahlberg, Water Sci. Technol., 1998, 37, 121 CrossRef CAS.
- G. Sogaard, R. Medenwaldt and V. Abraham-Peskir, Water Res., 2000, 34, 2675 CrossRef.
- Y. Saddeek, H. Shokry Hassan and G. Abd elfadeel, J. Non-Cryst. Solids, 2014, 403, 47 CrossRef CAS.
- T. Liu, Z. L. Wang, X. Yan and B. Zhang, Chem. Eng. J., 2014, 245, 34 CrossRef CAS.
- A. Baptista Neto, J. Smith and J. McAllister, Environ. Pollut., 2000, 109, 1 CrossRef PubMed.
- N. Asasian, T. Kaghazchi and M. Soleimani, J. Ind. Eng. Chem., 2012, 18, 283 CrossRef CAS.
- ATSDR, 2002, Public Health Service, Agency for Toxic Substances and Disease Registry, Subcontract No. ATSDR-205-1999-00024.
- EPA, Edition of the Drinking Water Standards and Health Advisories, 2011, http://www.water.epa.gov/action/advisories/drinking/upload/dwstandards2012.pdf Search PubMed.
- A. Volkovich, R. Griffiths and C. Thied, J. Nucl. Mater., 2003, 323, 49 CrossRef.
- M. Abdel-Aziz, I. Nirdosh and G. Sedahmed, Ind. Eng. Chem. Res., 2013, 52, 11655 CrossRef CAS.
- Y. Zhang, S. Zhang and T. Chung, Environ. Sci. Technol., 2015, 49, 10235 CrossRef CAS PubMed.
- H. Hamad, M. Abd El-latif, A. Kashyout, W. Sadik and M. Feteha, New J. Chem., 2015, 39, 3116 RSC.
- H. Hamad, M. Abd El-latif, A. Kashyout, W. Sadik and M. Feteha, Process Saf. Environ. Prot., 2015, 98, 390 CrossRef CAS.
- H. A. Hamad, W. Sadik, M. M. Abd El-latif, A. B. Kashyout and M. Y. Feteha, J. Environ. Sci., 2016, 43, 26 CrossRef CAS PubMed.
- M. F. Elkady and H. Shokry Hassan, Nanoscale Res. Lett., 2015, 10, 474 CrossRef CAS PubMed.
- Z. Elouear, J. Bouzid, N. Boujelben, M. Feki, F. Jamoussi and A. Montiel, J. Hazard. Mater., 2008, 156, 412 CrossRef CAS PubMed.
- R. M. Ali, H. A. Hamad, M. M. Hussein and G. F. Malash, Ecol. Eng., 2016, 91, 317 CrossRef.
- Y. Fenga, J. Gonga, G. Zenga, Q. Niua, H. Zhanga, C. Niua, J. Denga and M. Yan, Chem. Eng. J., 2010, 162, 487 CrossRef.
- A. Krestou, A. Xenidis and D. Panias, Miner. Eng., 2004, 17, 373 CrossRef CAS.
- Y. P. Xu, F. W. Schwartz and S. J. Traina, Environ. Sci. Technol., 1994, 28, 1472 CrossRef CAS PubMed.
- F. Foroughi, S. Hassan zadeh-Tabrizi, J. Amighian and A. Saffar-Teluri, Ceram. Interfaces, 2015, 41, 6844 CrossRef CAS.
- M. A. A. A. El-Remaily and H. A. Hamad, J. Mol. Catal. A: Chem., 2015, 404, 148 CrossRef.
- J. Hu, I. Lo and G. Chen, Sep. Purif. Technol., 2007, 56, 249 CrossRef CAS.
- S. Zhang, H. Qu, J. Liu, P. Liu and C. Wu, Water Res., 2007, 41, 1921 CrossRef PubMed.
- M. El-Aassar, M. F. El-Kady, H. Shokry Hassan and S. Al-Deyab, J. Taiwan Inst. Chem. Eng., 2016, 58, 274 CrossRef CAS.
- J. Hu, D. D. Shao, C. L. Chen, G. D. Sheng, J. X. Li, X. K. Wang and M. Nagatsu, J. Phys. Chem. B, 2010, 114, 6779 CrossRef CAS PubMed.
- N. Feng, X. Guo and S. Liang, J. Hazard. Mater., 2009, 164(2–3), 1286 CrossRef CAS PubMed.
- P. Chand and Y. Pakade, Environ. Sci. Pollut. Res., 2015, 22, 10919 CrossRef CAS PubMed.
- J. F. Cawthray, A. L. Creagh, C. A. Haynes and C. Orvig, Inorg. Chem., 2016, 54(4), 1440 CrossRef PubMed.
- F. Zhuang, R. Tan, W. Shen, X. Zhang, W. Xu and W. Song, J. Alloys Compd., 2015, 637, 531 CrossRef CAS.
- H. K. Boparai, M. Joseph and D. M. O'Carrol, J. Hazard. Mater., 2011, 186, 458 CrossRef CAS PubMed.
- L. Wu, W. Forsling and P. W. Schindler, J. Colloid Interface Sci., 1991, 147, 178 CrossRef CAS.
- J. Reichert and J. Binner, J. Mater. Sci., 1996, 5, 1231 CrossRef.
- M. S. Mohy-Eldin, M. F. Elkady, M. A. Abu-Saied, A. M. Abdel Rahman, E. A. Soliman, A. A. Elzatahry and M. E. Youssef, J. Appl. Polym. Sci., 2010, 118, 3111 CrossRef CAS.
- M. F. Elkady and H. Shokry Hassan, Curr. Nanosci., 2015, 11, 805 CrossRef CAS.
- L. Chen, J. Hu, D. Shao, X. Li and K. Wang, J. Hazard. Mater., 2009, 164, 923 CrossRef PubMed.
- A. Bhatnagar and A. K. Minocha, Colloids Surf., B, 2010, 76, 544 CrossRef CAS PubMed.
- S. Larous, A. H. Meniai and M. B. Lehocine, Desalination, 2005, 185, 483 CrossRef CAS.
- C. H. Hsieh, S. L. Lo, W. H. Kuan and C. L. Chen, J. Hazard. Mater., 2006, 136, 338 CrossRef CAS PubMed.
- Z. Wang, P. Yin, R. Qu, H. Chen, C. Wang and S. Ren, Food Chem., 2013, 136, 1508 CrossRef CAS PubMed.
- G. D. Sheng, J. Sheng, S. T. Yang, J. Hu and X. K. Wang, J. Radioanal. Nucl. Chem., 2011, 289(1), 129 CrossRef CAS.
- P. Mondal, C. Majumder and B. Mohanty, J. Hazard. Mater., 2008, 150, 695 CrossRef CAS PubMed.
- S. Mohan and R. Gandhimathi, J. Hazard. Mater., 2009, 169, 351 CrossRef CAS PubMed.
- K. Ajay, G. Mishra, P. Rai, R. Chitra and P. Nagar, J. Hazard. Mater., 2005, 122, 161 CrossRef PubMed.
- M. Dinesh and C. Subhash, J. Hazard. Mater., 2006, 137, 1545 CrossRef PubMed.
- K. Maria, Water Res., 2006, 40, 3167 CrossRef PubMed.
- M. F. Elkady, H. Shokry Hassan and E. EL-Sayed, J. Chem., 2015, 1–10 Search PubMed.
- C. Ng, J. N. Losso, W. E. Marshall and R. M. Rao, Bioresour. Technol., 2002, 85, 131 CrossRef CAS PubMed.
- H. Freundlich, Colloid and Capillary Chemistry, Methuen, London, UK, 1926 Search PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361 CrossRef CAS.
- M. J. Temkin and V. Pyzhev, Acta Physicochim. URSS, 1940, 12, 217 Search PubMed.
- M. F. Elkady, E. EL-Sayed, H. Farag and A. Zaatout, J. Nanomater., 2014, 1–11 CrossRef.
- T. Karthikeyan, S. Rajgopal and L. R. Miranda, J. Hazard. Mater., 2005, 124, 192 CrossRef CAS PubMed.
- Y. L. F. Musico, C. M. Santos and M. L. P. Dalida, J. Mater. Chem. A, 2013, 1, 3789 CAS.
- H. Shokry, M. F. Elkady, A. El-Shazly and H. Bamufleh, J. Nanomater., 2014, 1–14 CrossRef.
- S. T. Yang, J. X. Li, D. D. Shao, J. Hu and X. K. Wang, J. Hazard. Mater., 2009, 166, 109 CrossRef CAS PubMed.
- Y. Kim, C. Kim, I. Choi, S. Rengraj and J. Yi, Environ. Sci. Technol., 2004, 38, 924 CrossRef CAS PubMed.
- A. A. Khan and R. P. Singh, J. Colloid Interface Sci., 1987, 24, 33 CAS.
- W. S. Ngah and M. A. K. M. Hanafiah, Biochem. Eng. J., 2008, 39, 521 CrossRef.
- X. Hongqin, W. Duilin, J. Zhe, L. Xiaowei, Z. Shouwei, L. Yan and C. Cheng, J. Radioanal. Nucl. Chem., 2012, 292, 637 CrossRef.
- S. S. Tahir and N. Rauf, J. Chem. Thermodyn., 2003, 35, 2003 CrossRef CAS.
- P. Merike, T. Kaia and B. Villem, Environ. Sci. Technol., 2004, 38, 5626 CrossRef.
- Y. J. Wang, J. H. Chen, Y. X. Cui, S. Q. Wang and D. M. Zhou, J. Hazard. Mater., 2009, 162, 1135 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |