Study of cetyltrimethyl ammonium bromide and benzylideneacetone as electrolyte additives for valve-regulated lead-acid batteries under high-rate partial-state-of-charge conditions
L. Zhao*,
W. Zhou,
J. Z. Wu,
Q. Wu and
D. L. Wang
MIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, 150001, China. E-mail: dhx907@hit.edu.cn
First published on 23rd August 2016
Abstract
Cetyltrimethyl ammonium bromide and benzylideneacetone are used as electrolyte additives respectively for inhibiting hydrogen evolution on electrochemically active carbon and prolonging the cycle life of valve-regulated lead-acid batteries working under high-rate partial-state-of-charge conditions. It has been found that the addition of cetyltrimethyl ammonium bromide or benzylideneacetone in H2SO4 electrolyte can effectively increase the overpotential of hydrogen evolution and decrease the evolution rate of hydrogen on electrochemically active carbon. An appropriate addition amount of cetyltrimethyl ammonium bromide or benzylideneacetone in the H2SO4 electrolyte of batteries can remarkably slow down the evolution rate of hydrogen on the negative plates, decrease the cut-off charging voltage, and increase the cut-off discharging voltage. Furthermore, the presence of cetyltrimethyl ammonium bromide or benzylideneacetone prolongs the cycle life of valve-regulated lead-acid batteries under high-rate partial-state-of-charge conditions. In comparison with cetyltrimethyl ammonium bromide at the concentration of 240 mg L−1, benzylideneacetone at the concentration of 20 mg L−1 shows better performances in terms of the improved cycle life of the batteries.
Introduction
Lowering the energy consumption of automobiles has encouraged the use of various types of hybrid electric vehicles (HEVs). The battery in HEVs is charged and discharged at a very high frequency under high-rate partial-state-of-charge (HRPSoC) conditions.1 At present, the energy-storage candidate systems for HEVs mainly include valve-regulated lead-acid (VRLA) batteries, nickel–metal hydride (Ni–MH) battery, rechargeable lithium battery and supercapacitor. Among them, VRLA batteries has some advantages including well-established manufacturing base, low initial cost, and high recycling efficiency.2–4 Nevertheless, VRLA batteries operating under HRPSoC conditions result in the irreversible formation of lead sulfate in negative plates,1,5,6 which limits their cycle life.In order to solve such problem, carbon materials were added into the negative active material.7–14 The carbon materials could restrain the sulfation of the negative plates and inhibit capacity loss by competing with lead to react with O2 during charge.15–17 Carbon materials in H2SO4 solution can also act as a double-layer capacitor electrode, which serves as a buffer to share the charge and discharge currents with the lead-acid negative plates and thus prevents them being discharged and charged at the operational high rates.18 All these effects of the carbon materials contribute to the improved cycle life of VRLA batteries in HRPSoC cycling applications. In order to facilitate the electrochemical reactions of charge, the carbon material added to the negative plate should have the following characteristics: (a) high electroconductivity, (b) high specific surface area, and (c) strong adhesion to the lead surface. The carbon with the above characteristics is called as “electrochemically active carbon” (labelled as EAC).19
Although the addition of carbon materials in the negative plates can improve the cycle performance of VRLA batteries in HRPSoC applications, it will increase the evolution rate of hydrogen on the negative plates during charging process,20 which negatively influences the cycle performance of battery. Therefore, it is necessary to reduce the hydrogen evolution rate induced by the carbon materials during charging process. The addition of L-serine in electrolyte or the modification of carbon materials with diethylenetriamine can inhibit the hydrogen evolution on negative plates of VRLA batteries.21,22
In this paper, cetyltrimethyl ammonium bromide (CTAB) and benzylideneacetone were added in H2SO4 solution respectively for the purpose of inhibiting the hydrogen evolution on EAC and prolonging the HRPSoC cycle life of VRLA batteries.
Experimental
Preparation of negative plates
In this study, EAC negative electrodes and the negative plates of VRLA batteries were prepared, respectively. EAC negative electrodes were prepared by filling EAC (90%) and acetylene black (10%) with carboxymethyl cellulose (CMC) binder into Pb–Ca grids. Negative plates of VRLA batteries were prepared by using lead oxide powder, EAC, barium sulfate and humic acids by following a common industrial method.Preparation of electrolyte
H2SO4 solution with a density of 1.35 g cm−3 was used as electrolyte, and different content of CTAB or benzylideneacetone was added in H2SO4 electrolyte. The content of CTAB was varied from 0 to 320 mg L−1, and the content of benzylideneacetone was controlled at 0, 20 mg L−1, 40 mg L−1, 60 mg L−1 and 80 mg L−1, respectively.Performance measurements
The effects of electrolyte additives on the evolution rate of hydrogen were studied by using linear sweep voltammetric method. A prepared negative plate was used as working electrode, a commercial PbO2 positive plate and a Hg/Hg2SO4 electrode were used as counter electrode and reference electrode, respectively. H2SO4 solution added with CTAB or benzylideneacetone was used as electrolyte. The measuring equipment was a CHI430A electrochemical workstation made by CH Instruments Inc. The scanning range was −0.35 V to −1.45 V, and scanning rate was 10 mV s−1.Galvanostatic charge–discharge cycling was performed on a BTS series battery testing system which is made by Neware Technology to investigate the effects of electrolyte additives on the cycle performance of VRLA batteries under HRPSoC conditions. Test cells were assembled from two commercial positive plates and one prepared negative plate. H2SO4 solution added with CTAB or benzylideneacetone was used as the electrolyte. After being activated, the test cells were firstly discharged to 50% of their initial capacity at 1C rate and then cycled as follows: charge at 2.5C rate for 60 s, stand for 60 s, discharge at 2.5C rate for 60 s, stand for 60 s. The test process was terminated automatically when the cut-off discharging voltage of the test cells fell down to 1.60 V or the cut-off charging voltage increased to 2.90 V.
Phase structures of the negative plates were characterized by powder X-ray diffraction, Rigaku D/MAX-rB, Cu Kα radiation at 45 kV.
Results and discussion
Effects of hexadecyl trimethylammonium bromide on the cathodic process of electrochemically active carbon
Fig. 1 shows the linear sweep curves of EAC in H2SO4 solution added with different contents of CTAB. It is obviously found that the CTAB added in H2SO4 electrolyte can influence the cathode current on EAC. A current peak was observed at the potential of about −1.20 V, which corresponds to the reduction reaction of PbSO4 (produced by the dissolution of grids in H2SO4 solution) to Pb. In the potential range of −0.4 V to −1.1 V, the current corresponding to certain potential is the charge current of electrical double layer, which reflects the capacity of electrical double layer capacitor. For the potential lower than −1.1 V, the current consists of two parts: the current of electrical double layer charge and the one of hydrogen evolution. It can be seen that with increasing CTAB content in H2SO4 electrolyte, the charge current of electrical double layer decreased slightly, while the current of hydrogen evolution decreased obviously. This indicates that increasing CTAB content in H2SO4 electrolyte could decrease the hydrogen evolution rate on EAC. In Fig. 1, at the greatest negative potential, the curves for the 0 mg and 80 mg cases crossed over. The phenomenon is mainly due to the capacitance of the electrical double layer. At the potential of −1.4 V, the content of CTAB absorbed on the surfaces of the electrode is notably increased, resulting in the enhanced capacitance and charging current in comparison to the electrode without CTAB modification. It can also be seen from Fig. 1 that further addition of CTAB in H2SO4 electrolyte (e.g. 320 mg L−1) will decrease the charge current of electrical double layer of EAC, that will lead to the worse performances of the batteries. | ||
| Fig. 1 Linear sweep curves of EAC in H2SO4 electrolyte added with different contents of CTAB at a scan rate of 10 mV s−1. | ||
In order to clarify the effects of CTAB in H2SO4 electrolyte on the hydrogen evolution rate, in this study, a steady-state method was used to eliminate the disturbance of the charge current of electrical double layer. The steady hydrogen evolution curves of EAC in H2SO4 electrolyte added with different contents of CTAB were given in Fig. 2. It can be seen from Fig. 2 that CTAB added in H2SO4 electrolyte can influence obviously the steady hydrogen evolution rate. With increasing the content of CTAB in H2SO4 electrolyte, the hydrogen evolution rate decreased. However, when the concentration of CTAB is lower than 240 mg L−1, the hydrogen evolution rate on EAC electrode unexpectedly raised higher than the one without CTAB. CTAB molecule contains Br− and NH4+. Br− can facilitate the cathodic process, while NH4+ is able to suppress it. When the amount of CTAB used is low (i.e. 80 mg), the content of CTAB absorbed on the surface of the electrode is low. In this case, the positive effects of Br− on the cathodic process are over than the negative effects of NH4+. As a result, the hydrogen evolution currents on the surfaces of the carbon electrode are significantly improved. Comprehensively considering the effects of CTAB on both hydrogen evolution rate and charge current of electrical double layer, an appropriate concentration of CTAB at 240 mg L−1 CTAB in H2SO4 electrolyte was used in following experiments.
 | ||
| Fig. 2 Steady hydrogen evolution curves of EAC in H2SO4 electrolyte added with different contents of CTAB. | ||
Effects of benzylideneacetone on the cathodic process of electrochemically active carbon
Fig. 3 shows the linear sweep curves of EAC in H2SO4 solution added with different contents of benzylideneacetone. In the potential range of −0.4 V to −1.1 V, the current corresponding to certain potential decreased with increasing benzylideneacetone content in H2SO4 electrolyte, reflecting that the appropriate amount of benzylideneacetone could regulate the capacity of electrical double layer capacitor. In the potential range of −1.10 V to −1.50 V, except the reduction current peak of PbSO4 to Pb at about −1.20 V, there occurred the current of double layer charge and hydrogen evolution as well. In the current measurement, the scan rate for each electrode was the same (10 mV s−1), that is, the current of double layer charge for each electrode is almost the same. Therefore the current difference for each electrode mainly results from the different hydrogen evolution. Therefore, it can be seen that increasing benzylideneacetone content in H2SO4 can decrease the hydrogen evolution rate. Based on an overall consideration of the effects of benzylideneacetone on the capacity of electrical double layer capacitor and the rate of hydrogen evolution, an appropriate amount of benzylideneacetone should be added in H2SO4 electrolyte. | ||
| Fig. 3 Linear sweep curves of EAC in H2SO4 electrolyte added with different contents of benzylideneacetone at a scan rate of 10 mV s−1. | ||
In order to explore the hydrogen evolution behavior of EAC in H2SO4 electrolyte added with benzylideneacetone, the corresponding steady hydrogen evolution curves were measured and given in Fig. 4. As can be seen, since the charge current of the electrical double layer was eliminated, the current value represents the rate of hydrogen evolution of EAC, which could be adjusted by addition of benzylideneacetone in H2SO4 electrolyte. At a given potential, the hydrogen evolves on the EAC electrode without benzylideneacetone in H2SO4 electrolyte was more than those on the EAC electrode with benzylideneacetone in H2SO4 electrolyte. Further increasing benzylideneacetone content in H2SO4 electrolyte resulted in the obviously decreased hydrogen evolution rate. Therefore, it can be concluded that the addition of benzylideneacetone in H2SO4 electrolyte can decrease the hydrogen evolution rate on EAC effectively. Comparing the curves shown in Fig. 3 and 4, it can be found that the current in Fig. 3 is bigger than that of in Fig. 4, which is due to the charge current of electrical double layer in Fig. 3. Therefore, it can be deduced that the EAC electrode in H2SO4 electrolyte modified with benzylideneacetone has supercapacitor property, which is beneficial to the performance of the electrodes in the batteries. However, a large amount of benzylideneacetone in H2SO4 electrolyte will decrease the charge current of the electrical double layer of EAC. Considering the effects of benzylideneacetone on both hydrogen evolution rate and charge current of electrical double layer, an optimized concentration of 20 mg L−1 for benzylideneacetone in H2SO4 electrolyte was used in following experiments.
 | ||
| Fig. 4 Steady hydrogen evolution curves of EAC in H2SO4 electrolyte added with different contents of benzylideneacetone. | ||
CTAB and benzalacetone are commonly used in electroplate to enhance the overpotential of the cathodic process. CTAB and benzalacetone added in VRLA batteries are absorbed onto the surfaces of the cathode during the charging process, and thus enhance the overpotential of the cathodic process and reduce the hydrogen evolution rate during the charging process through the synergistic effects of ψ1 effect, enclosure effects, penetration effect and Heyrovsky effects.
Effects of electrolyte additives on the hydrogen evolution behavior of the negatives in VRLA batteries
According to the above findings, 240 mg L−1 CTAB or 20 mg L−1 benzylideneacetone in H2SO4 electrolyte were used as the additive for the electrolyte of VRLA batteries to decrease the hydrogen evolution rate. In order to ensure the reliability of the experimental results, before measurement, the prepared negatives of VRLA batteries were firstly activated and then were charged with 0.1C to 2.42 V, and maintained this potential for another 10 h to make them in a state of charge of 100%.Fig. 5 shows the cathodic polarization curves of the negative plates of VRLA batteries in H2SO4 electrolyte added with different additives at a scan rate of 10 mV s−1. It can be seen from Fig. 5 that four curves present a similar changing trend when the potential was higher than −1.25 V. This could be due to a reaction of the reduction of PbSO4 in the negatives under the potential higher than −1.25 V. As mentioned above, all the negatives of VRLA batteries were in a state of 100% SOC before testing, no reduction current peak of PbSO4 was detected because of no existence of PbSO4. When the potential is lower than −1.25 V, hydrogen began to evolve on the electrodes to rapidly increase the current.
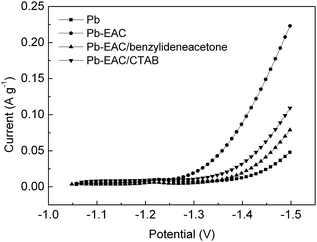 | ||
| Fig. 5 Cathodic polarization curves of the negative plates of VRLA batteries in H2SO4 electrolyte added with different additives at a scan rate of 10 mV s−1. | ||
As shown in Fig. 5, the Pb negative exhibits the minimum hydrogen evolution rate; while Pb added with EAC (Pb–EAC) presents an obviously increased hydrogen evolution rate. In addition, CTAB or benzylideneacetone added in H2SO4 electrolyte can decrease the hydrogen evolution rate, in detail, 20 mg L−1 benzylideneacetone in H2SO4 electrolyte has more notable effects on restraining hydrogen evolution than 240 mg L−1 CTAB in H2SO4 electrolyte.
In order to eliminate the disturbance of electrical double layer charge, the steady hydrogen evolution curves of the negative plates in H2SO4 electrolyte added with different contents of the additives were measured and given in Fig. 6. It can be seen from Fig. 6 that, for all negative plates, no obvious current associated with the reduction of PbSO4 to Pb was found at the potential of −1.25 V, which is well consistent with the findings shown in Fig. 5. Hydrogen was produced as the potential was lower than −1.25 V. The addition of AC in Pb negative plate increased hydrogen evolution rate remarkably. Hydrogen evolution is a harmful side-reaction for VRLA batteries, and it causes the electrolyte dryout. Some precaution must be taken to suppress the hydrogen evolution. It can be seen from Fig. 6 that the addition of CTAB or benzylideneacetone in H2SO4 electrolyte decreases the evolution rate of hydrogen on the negative plates. CTAB and benzylideneacetone can be easily adsorbed onto AC to obstruct the formation of atomic hydrogen in H2SO4 solution. As a result, the hydrogen evolution on AC during cathodic polarization process is suppressed. In comparison to 20 mg L−1 benzylideneacetone in H2SO4 electrolyte, 240 mg L−1 CTAB in H2SO4 electrolyte exhibits more notable effects on restraining hydrogen evolution.
 | ||
| Fig. 6 Steady hydrogen evolution curves of the negative plates in H2SO4 electrolyte added with different contents of additives. | ||
Effects of electrolyte additives on HRPSoC performance of VRLA batteries
HEVs require the battery to be charged and discharged under HRPSoC conditions. In this study, the effects of the electrolyte additives on the cycle performance of VRLA batteries under HRPSoC conditions were investigated. Fig. 7 gives the cycle performance of VRLA batteries under HRPSoC conditions after addition with benzylideneacetone and CTAB in H2SO4 electrolyte. It can be seen from Fig. 7 that the batteries added with EAC in the negative plates showed a stable cut-off charging voltage and a lower cut-off discharging voltage than that without EAC. In general, during HRPSoC cycle process, the cut-off voltage of VRLA batteries changes constantly. When the cut-off charging voltage increased to 2.90 V or the cut-off discharging voltage decreased to 1.60 V, the cycle process was terminated consequently. As shown in Fig. 7, the cut-off charging voltages of the batteries with Pb negatives increased rapidly after three hundred cycles and reached 2.90 V in the 380th cycles, while the cut-off charging voltages of the batteries with the negative plates added with EAC remained unchanged within a certain range. The cut-off discharging voltages of all batteries decreased gradually with increase of cycle numbers. It also can be seen from Fig. 7 that the addition of EAC in negative plates prolongs the cycle life of VRLA batteries and changed the reasons of cycle life termination. The cycle life termination for batteries with Pb negative plates could be due to the fact that the cut-off charging voltages reach the highest setting limit of voltage 2.90 V, while the termination for batteries with the negative plates added with EAC is due to the fact that the cut-off discharging voltages decrease to the lowest setting limit of voltage 1.60 V. The addition of EAC in the negative plates could restrain the sulfation of negative plates, and provide conductive network to facilitate charging process.23 Carbon materials can act as a double-layer capacitor electrode, which serves as a buffer to share the charge and discharge currents with the lead-acid negative plates and thus prevents them from being discharged and charged at the high rates. So the addition of EAC in negative plates prolongs the cycle life of batteries and lowers the cut-off charging voltages. | ||
| Fig. 7 Cut-off charging/discharging voltage of batteries contained different negative plates and electrolyte additives during cycle test process. | ||
The electrolyte additives play a very important role in prolonging the cycle life of VRLA batteries with the negative plates added with EAC under HRPSoC conditions. The addition of benzylideneacetone in H2SO4 electrolyte prolonged the cycle life of VRLA batteries by 20%, while the addition of CTAB prolonged the cycle life by 100%. Moreover, the addition of CTAB in H2SO4 electrolyte enhanced the cut-off discharging voltages. For all VRLA batteries with the negative plates added with EAC, the cut-off discharging voltages decreased gradually with increase of cycle numbers till the lowest setting limit of voltage 1.60 V, while the cut-off charging voltages did not reach the highest setting limit of voltage 2.90 V. This phenomenon indicates that the additives in H2SO4 electrolyte have an important influence on the charge acceptance of the batteries. The cycle life of battery is measured as follow: charging at 2.5C rate for 60 s and standing for 60 s, discharging at 2.5C rate for 60 s and standing for 60 s. The electric quantity of charge and discharge is same in a single cycle. The battery with a higher charge acceptance could be charged with more effective electric quantity, and it thus shows a higher cut-off discharging voltage during the discharge process. Therefore, the battery with 240 mg L−1 CTAB in H2SO4 electrolyte has the highest charge acceptance.
The electrolyte additives have a great influence on HRPSoC cycle performance of VRLA batteries. In order to clarify the effects of electrolyte additives on HRPSoC cycle performance of the VRLA batteries, the chemical composition of the negative plates were investigated. Fig. 8 presents the XRD patterns of the negative plates of VRLA batteries after HRPSoC cycle tests. The characteristic peaks of Pb are identified with a sign, and the other characteristic peaks are assigned to PbSO4. All XRD patterns show similar profiles, showing major peaks at same locations and with different intensities. With adding EAC in the negative plates, the intensities of the characteristic peaks of Pb increased and those of PbSO4 decreased. This phenomenon indicates that the addition of EAC in the negative plates can facilitate conversion of PbSO4 to Pb to prevent the accumulation of PbSO4 on the negative plates. As a result, the cycle performance of VRLA batteries under HRPSoC conditions was improved. It also can be seen from Fig. 8 that the addition of CTAB or benzylideneacetone in H2SO4 electrolyte has no obvious influence on the characteristic peaks of Pb and PbSO4. This phenomenon suggests that the addition of CTAB or benzylideneacetone can mainly influence the hydrogen evolution rate of negative plates in the charging process instead of the conversion between PbSO4 and Pb.
Conclusions
The addition of CTAB or benzylideneacetone in H2SO4 electrolyte can effectively increase the overpotential of hydrogen evolution and decrease the evolution rate of hydrogen on EAC. An appropriate amount of CTAB or benzylideneacetone in H2SO4 electrolyte of VRLA batteries can remarkably decrease the evolution rate of hydrogen on the negative plates, and improve the cycle performance of VRLA batteries under HRPSoC conditions. By comparison with benzylideneacetone, CTAB is more effective. The battery added with 240 mg L−1 CTAB in H2SO4 electrolyte exhibits the longer cycle life than that with 20 mg L−1 benzylideneacetone.Acknowledgements
This work was supported by Tianneng Group.Notes and references
- L. T. Lam and R. Louey, J. Power Sources, 2006, 158, 1140 CrossRef CAS.
- J. Albers, E. Meissner and S. Shirazi, J. Power Sources, 2011, 196, 3993 CrossRef CAS.
- H. Li, H. P. Liu, Q. M. Wang, H. Y. Chen, A. F. Ren and J. G. Hu, Electrochim. Acta, 2010, 56, 663 CrossRef CAS.
- D. Pech, T. Brousse, D. Bélanger and D. Guay, Electrochim. Acta, 2009, 54, 7382 CrossRef CAS.
- L. T. Lam, N. P. Haigh, C. G. Phyland and A. J. Urban, J. Power Sources, 2004, 133, 126 CrossRef CAS.
- L. T. Lam, N. P. Haigh, C. G. Phyland and T. D. Huynh, J. Power Sources, 2005, 144, 552 CrossRef CAS.
- D. P. Boden, D. V. Loosemore, M. A. Spence and T. D. Wojcinski, J. Power Sources, 2010, 195, 4470 CrossRef CAS.
- D. Pavlov, P. Nikolov and T. Rogachev, J. Power Sources, 2010, 195, 4444 CrossRef CAS.
- D. Pavlov, P. Nikolov and T. Rogachev, J. Power Sources, 2011, 196, 5155 CrossRef CAS.
- L. T. Lam, R. H. Newnham, H. Ozgun and F. A. Fleming, J. Power Sources, 2000, 88, 92 CrossRef CAS.
- P. Bača, K. Micka, P. Křivík, K. Tonara and P. Tošer, J. Power Sources, 2011, 196, 3988 CrossRef.
- P. T. Moseley, R. F. Nelson and A. F. Hollenkamp, J. Power Sources, 2006, 157, 3 CrossRef CAS.
- M. Fernández, J. Valenciano, F. Trinidad and N. Muñoz, J. Power Sources, 2010, 195, 4458 CrossRef.
- S. M. Kumar, S. Ambalavanan and S. Mayavan, RSC Adv., 2014, 4, 36517 RSC.
- K. Nakamura, M. Shiomi, K. Takahashi and M. Tsubota, J. Power Sources, 1996, 59, 153 CrossRef CAS.
- M. Calabek, K. Micka, P. Krivak and P. Baca, J. Power Sources, 2006, 158, 864 CrossRef CAS.
- K. K. Yeung, X. F. Zhang, S. C. T. Kwok, F. Ciucci and M. M. F. Yuen, RSC Adv., 2015, 5, 71314 RSC.
- A. Cooper, J. Furakawa, L. Lam and M. Kellaway, J. Power Sources, 2009, 188, 642 CrossRef CAS.
- D. Pavlov, T. Rogachev, P. Nikolov and G. Petkova, J. Power Sources, 2009, 191, 58 CrossRef CAS.
- Y. Chen, B. Z. Chen, X. C. Shi, H. Xu, W. Shang, Y. Yuan and L. P. Xiao, Electrochim. Acta, 2008, 53, 2245 CrossRef CAS.
- M. A. Deyab, RSC Adv., 2015, 5, 41365 RSC.
- B. Hong, X. Y. Yu, L. X. Jiang, H. X. Xue, F. Y. Liu, J. Li and Y. X. Liu, RSC Adv., 2014, 4, 33574 RSC.
- M. Shiomi, T. Funato, K. Nakamura, K. Takahashi and M. Tsubota, J. Power Sources, 1997, 64, 147 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |

