Rapid and facile synthesis of high-quality, oleate-capped PbS nanocrystals†
W. Ryan Tilluck,
Alexander L. Morris,
Jason K. Gurchiek,
Amanda D. Evans and
P. Gregory Van Patten*
Department of Chemistry, Middle Tennessee State University, Murfreesboro, TN 37132, USA. E-mail: greg.vanpatten@mtsu.edu
First published on 19th August 2016
Abstract
A method for producing PbS nanocrystals using air stable precursors is examined and compared to existing methods. The method employs thermal decomposition of thiocarbonyls to produce the reactive sulfide needed to react with lead oleate. Thioacetamide is emphasized in this work, but similar results are obtained with other thioamides and with various thioureas. The use of these reagents in the synthesis of PbS NCs is not new; however, this is the first detailed examination of the potential to produce high quality PbS nanocrystals by these methods, and new insights on this approach are presented here. Using thioacetamide in combination with lead oleate and a mixture of solvents, we have obtained PbS nanocrystals with relatively narrow (≤60 meV) exciton absorption peaks, moderate photoluminescence quantum yield, and good stability against size change or degradation upon storage. Size tuning over a wide range—from 4 nm to 10 nm particle diameters—can be achieved by controlling precursor concentrations or by gradually injecting multiple precursor doses. This allows for tuning of the lowest exciton energy across the near infrared from less than 0.60 eV to 1.0 eV.
Introduction
The search for an ideal synthetic route to PbS nanocrystals (NCs), also known as quantum dots, has continued for over a decade. The electronic structure of PbS NCs gives rise to intense optical transitions that are tunable across the near infrared spectral region and even into the visible range. This spectral range is important for many applications including wired, high-speed communications, night vision, biomedical imaging and labeling, and solar energy conversion. Numerous PbS synthesis routes have been reported, each with its own characteristic advantages. Among these published routes, a few have been adopted widely. To justify widespread adoption, a particular route must yield NCs within a useful range of sizes (mean size between approximately 2.5 and 10 nm), must provide some degree of size tuning and control, must produce particles that are relatively monodisperse (<10% standard deviation in particle diameter), and must give at least moderate photoluminescence quantum yield (PLQY).In 2003, Hines and Scholes reported the first synthesis of high quality PbS NCs.1 Their method used a highly reactive sulfur precursor, hexamethyldisilathiane (HMDS). Drawbacks associated with HMDS include its air sensitivity, toxicity, volatility, and extreme malodor. Nevertheless, that procedure allows size tuning over a wide range with moderate size distribution and also produces a modest PLQY of 15–20%. Hines and Scholes noted significant ripening of the particles as they aged at room temperature over a period of a few days. This is a notable and common property of PbS NCs: they exhibit relatively poor long-term stability in their size distribution. Generally, PbS NCs are observed to shrink over time, with smaller particles being generally more susceptible than larger ones. However, even large particles eventually begin to redissolve with time.
A few years later, Cademartiri et al. reported a route to PbS NCs with much higher yield than the Hines/Scholes reaction.2 Instead of using HMDS as a sulfur precursor, Cademartiri used elemental sulfur dissolved in oleylamine. The oleylamine reduces the sulfur to produce reactive sulfide and also serves as a ligand to coat the PbS NCs. This method produces very large batches of NCs with tunable size, and the particles reportedly resist etching when stored in air for long periods of time. In addition, the method is highly scalable and can be used to easily produce gram scale quantities of PbS QDs.
Cademartiri's production of high quality PbS NCs using sulfur in oleylamine instead of HMDS represented a significant advance because these reagents can be stored, handled, and reacted safely and easily even by less experienced hands. These chemicals are stable under atmospheric conditions for long periods, and unlike HMDS, their accidental release is not likely to produce noxious fumes. Synthesis of high quality semiconductor NCs often requires the use of air-free techniques, including vacuum/inert gas manifolds and inert atmosphere glove boxes in order to rigorously exclude oxygen and water from reaction mixtures; however, the elimination of highly air-sensitive and/or potentially hazardous reagents remains a significant advantage when possible.
The drawbacks of the Cademartiri approach are that the resulting particles are capped in oleylamine which reduces PLQY and that the residual PbCl2 left after synthesis continues to precipitate out over long periods of time, making it difficult to produce highly pure PbS NCs. Finally, the resulting particles are non-luminescent, size tuning is somewhat limited, and reproducibility of synthesis and purification are imperfect.
Moreels et al. found that adding tri-n-octylphosphine (TOP) to the Cademartiri reaction mixture could affect the final size distribution of the NCs and could significantly extend the size and wavelength range covered by that synthetic method.3 In that work, the authors showed that the TOP acted through formation of a low-reactivity tri-n-octylphosphine sulfide adduct, but the TOP was not found to bind to the surfaces of the product NCs. Like Cademartiri, these authors found that their NCs were quite stable against etching and shrinking, and they attributed this stability to the presence of surface-bound chloride ions.
Weidman et al. improved on the Cademartiri synthesis by using highly Pb-rich reaction mixtures and a novel purification process.4 The Weidman method produces highly monodisperse samples, though this is achieved through a novel, multi-step purification procedure that includes ligand exchange to replace the surface-bound oleylamine with oleic acid, thus imparting photoluminescent properties to these NCs. High quantum yields, up to 60%, can be attained with these particles. Like the Cademartiri NCs, these NCs showed exceptional stability against shrinkage when stored as a dry powder in air.
The Weidman method produces the highest quality PbS NCs reported to date. The monodispersity, high quantum yield, and long-term stability are exceptional among PbS NC materials. In principle, the method can be scaled up, too; however, it is worth noting that the reported product yield relative to the PbCl2 precursor is only about 2.5%. The primary drawback of this method is that, like the Cademartiri method, the unreacted PbCl2 continues to slowly precipitate for a period of days after synthesis, making quick production of highly pure NCs difficult. In addition, the mechanism by which this method achieves its remarkable monodispersity is not settled. Though the authors of that work did not intentionally employ size selective precipitation, recent work in our laboratory points to size selection as a byproduct of their purification process.5
Recently, Hendricks et al. reported the preparation of metal sulfide nanocrystals using a library of thiourea derivatives as the sulfide precursor.6 With their method, size tuning was achieved by altering the side chains of the urea derivatives to effect different levels of precursor reactivity. Changing the precursor reactivity also necessitated changing the reaction temperature, but broad size tunability was demonstrated in that report. Like the Cademartiri method, the Hendricks approach is highly scalable and can produce gram-scale quantities under readily achievable conditions. The only caveat with scale-up is that the final particle size attained is a function of precursor concentration as well as precursor identity and reaction temperature. For this reason, the optimal precursor and temperature for large batch synthesis will differ for large- and small-batch production of the desired NCs.
An interesting part of the Hendricks report is the description of a method to produce rigorously dry lead oleate using lead trifluoroacetate as an intermediate. In the more typical approach, PbO and oleic acid are heated to produce lead oleate and water. The PbO/oleic acid reaction is usually performed under vacuum at elevated temperatures, with the expectation that the water would be driven off under these conditions. However, Zherebetskyy et al. showed that residual hydroxide remains tightly bound to the metal oleate even under those conditions, and this bound hydroxide affects the surface chemistry and possibly long-term stability of the NCs.7 The Hendricks approach eliminates the possibility that trace water and/or hydroxide might remain and affect the chemistry of the NCs.
We have been inspired by the recent Hendricks publication to publish our studies of a closely related synthesis method that also uses a small thiocarbonyl, namely thioacetamide, as the sulfur precursor. This method is based on a method originally reported by Nagel et al.8–10 In their original reports, the synthetic method was ancillary to the main idea, so those reports did not include any discussion of the factors that influence quality, stability, and size distribution of the PbS NCs, nor did they include any reports of photoluminescence. Moreover, there are no subsequent published reports of the method from those authors or any others. Our group has refined the method to produce PbS NCs using only inexpensive, air-stable precursors and without need for complex purification procedures. The resulting NCs are luminescent, with PLQY values similar to those obtained using HMDS. These NCs are also stable, lasting up to one month with no noticeable changes in spectral properties. Although TAA has previously been used to produce metal sulfide nanostructures,11–15 there are no reports describing the production of high quality, luminescent PbS from TAA.
Experimental
Materials
Lead(II) oxide (ReagentPlus powder, >99.9%), 1-octadecene (ODE, technical grade, 90%), oleylamine (technical grade, 70%), thioacetamide (TAA, ACS reagent grade, ≥99.0%), N,N-dimethylformamide (DMF, anhydrous, 99.8%), and diphenyl ether (DPE, ReagentPlus, ≥99%) were obtained from Sigma-Aldrich and used as received except as explained below. Tetrachloroethylene (TCE, 99%, extra pure), oleylamine (OlAm, ∼80–90% C18 content), and PbO (99.9+%) were obtained from Acros Organics and used as received. Oleic acid (HOlAc, technical grade, 90%), and PbO (Puratronic, 99.999% metals basis) were obtained from Alfa Aesar and used as received, except as explained in the following procedures. Tri-n-octylphosphine (TOP, min. 97%) was obtained from Strem Chemicals and used as received. Absolute ethyl alcohol (ACS/USP grade) was obtained from Pharmco-Aaper and used as received.Synthesis of PbS nanocrystals
PbS nanocrystals (NCs) were prepared by rapidly injecting a solution of TAA into a solution of lead oleate (Pb(OlAc)2) at elevated temperature and under air-free conditions. Final particle size was dictated through control of precursor concentrations.Detailed procedures for producing particles of several different sizes are given in the ESI.†
In a typical synthesis, lead oleate was first prepared by combining 335 mg (1.5 mmol) PbO with 1.55 g (5.5 mmol) HOlAc and 5.0 mL of ODE in a 25 mL, three-necked round bottom flask. A magnetic stir bar was added, after which one side neck of the flask was equipped with a thermometer, the middle neck was connected to a reflux condenser and inert gas/vacuum manifold, and the remaining side neck was sealed with a septum. The flask was thoroughly evacuated (P < 0.1 Torr) and refilled with flowing, high-purity N2 gas. Next, 3.0 mL of TOP, taken from an inert gas glovebox in a sealed syringe, was injected though the septum under the flowing N2 gas. This mixture was heated with stirring to 95 °C under vacuum for 1 hour, until a clear solution of lead oleate was obtained. At that time, the flask was placed back under N2 flow, and the temperature was lowered to 85 °C.
To prepare the sulfur precursor, 40 mg (∼0.5 mmol) of TAA was thoroughly dissolved in 0.25 mL of DMF. After the TAA dissolved completely, 3.0 mL of TOP and 2.0 mL DPE were added, and the mixture was stirred until thoroughly mixed, approximately 10 minutes. This mixture was removed from the glovebox in a sealed, gas-tight syringe and rapidly injected into the stirring lead mixture at 85 °C. After the injection, the reaction was held at 85 °C for about one hour. To monitor reaction progress, small (0.2 mL) aliquots were taken at various times and purified according to the procedure described below.
In a modified version of this synthetic procedure, rigorously dry Pb(OlAc)2 that is free of excess HOlAc was prepared ahead of time following the method reported by Hendricks et al.6 This Pb(OlAc)2 source was also used in conjunction with intentionally added HOlAc and water to see if the effects of those additives on the reaction could be isolated, but interestingly, those reactions did not produce monodisperse PbS QDs.
This reaction has also been run with high purity HOlAc (≥99%) in place of technical grade. Identical results were obtained with both grades as long as the 95 °C vacuum distillation was run for one hour as described above.
Purification
The PbS NCs were separated from the reaction mixture through flocculation and centrifugation. One part of the crude reaction mixture was mixed with four parts ethanol by volume, and one part chloroform was then added to promote mixing. This mixture was vortexed for a few seconds to ensure complete mixing and then centrifuged at 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 3 minutes. The clear, colorless supernatant was discarded, and the resulting solid residue could be readily dispersed in non-polar solvents such as chloroform, toluene, hexanes, or TCE. A second purification step was conducted by dissolving the solid in a minimum amount of chloroform and adding ethanol or methanol to flocculate again. The solubility of the NCs decreased as additional purification cycles were used.
000g for 3 minutes. The clear, colorless supernatant was discarded, and the resulting solid residue could be readily dispersed in non-polar solvents such as chloroform, toluene, hexanes, or TCE. A second purification step was conducted by dissolving the solid in a minimum amount of chloroform and adding ethanol or methanol to flocculate again. The solubility of the NCs decreased as additional purification cycles were used.
Characterization
NC samples were suspended in TCE for spectroscopic measurements. UV/visible/NIR absorption and photoluminescence (PL) spectra were collected using an updated Cary 14 spectrophotometer (OLIS, Inc.). The instrument was fitted with an emission monochromator and a Peltier-cooled, amplified InGaAs photodiode. Excitation for PL measurements was achieved with a 50 mW diode laser operating at a wavelength of 980 nm. PL quantum yield was estimated by comparing total fluorescence intensity with that from a known NIR quantum yield standard,16 IR-26. IR-26 was dissolved at low concentration in dichloroethane, and the quantum yield of the standard was taken to be 0.06%.17,18 Fluorescence spectra of the standard, dissolved in dichloroethane (DCE), and the NC samples, dissolved in tetrachloroethylene (TCE), were collected at 90° geometry and corrected for wavelength-dependence of detector response as well as for re-absorption by the DCE solvent as discussed by Hatami et al.18 No efforts were made to protect the NCs from air or to evaluate changes in PLQY with time.18 Spectra were plotted and integrated against photon energy to quantify total emission intensity.Samples for TEM were prepared by diluting purified NCs in toluene. A drop of the dilute solution was applied to a carbon-coated copper TEM grid, and allowed to dry. Images were collected on a Hitachi H-7650 operating at 100 kV accelerating voltage or on a FEI Tecnai Osiris HRTEM operating at 200 kV.
Results and discussion
While this method resembles some other PbS nanocrystal (NC) synthesis procedures, the early kinetics of this reaction differ from most other hot injection reactions.19,20 In most cases, the precursor injection results in nearly instantaneous nucleation to produce rapidly-growing NCs. The presence of the growing NCs is generally evident from color changes in the reaction mixture. The present reaction differs from other cases in that the injection of the thioacetamide (TAA) precursor is often followed by a period of up to 3 minutes during which the reaction mixture does not appear to change at all. After this induction period, the reaction then rapidly darkens over a period of approximately 1 minute, as the lowest energy absorption peak begins to rapidly shift toward its final wavelength. Particle growth slows, but the absorption spectrum continues to evolve for another 10–15 minutes as the particle size approaches its final value. A typical reaction progression is shown in the images of Fig. 1, which document the first 3+ minutes of a PbS NC synthesis. The changes that occur in the solution during this induction period are not yet known, but several observations lend some insight. For example, despite the apparently slow progression at the reaction outset, the speed of injection remains a key factor in obtaining a narrow size distribution. A slightly-too-slow injection produces a much broader size distribution than a very fast injection; if the injection takes more than 2–3 seconds, multiple particle sizes can be distinguished in the absorption spectrum of the product. The importance of a fast injection implies that the metal and chalcogen precursors react rapidly—as fast as mixing occurs—to produce a colorless, reactive species that eventually leads to particle nucleation and growth. This situation is consistent with a La Mer-type growth process in which a rapid nucleation burst is followed by a much slower, and temporally distinct, particle growth phase.21Powder X-ray diffraction data from a sample of purified and dried nanocrystals is shown in Fig. 2. For comparison, the known diffraction pattern of the rock salt phase of PbS (galena) is also depicted, and the close match confirms the reaction product is PbS.
 | ||
| Fig. 2 Powder X-ray diffraction from a sample of the PbS nanocrystals (blue solid curve). The dashed vertical bars show the positions of reflexes for the rock salt phase of PbS. | ||
Fig. 3 shows absorption spectra collected at various times after injection of TAA into the Pb(OlAc)2 mixture at 85 °C. The data show that the lowest energy absorption peak red shifts as the reaction proceeds. The shift is rapid within the first ten minutes of the reaction, but then the peak position stabilizes after this time. The peak shape continues to evolve over time, with very slight changes in peak width. The peak narrows for the first 30–45 minutes and then begins to slowly broaden again, indicating the onset of Ostwald ripening. Efforts have been made to spectroscopically analyze the reaction trajectory at early times (<5 min) by collecting and purifying small aliquots. Unfortunately, these samples change visibly after collection, even when cooled rapidly to room temperature. For example, aliquots taken from a colorless reaction mixture may darken within seconds after collection and dilution either in a solvent (chloroform, toluene) or an anti-solvent (ethanol, methanol). Similarly, aliquots collected at a point in time when the reaction mixture appears red will turn green/brown upon collection and dilution. These observations call into question the assumption that aliquots collected even at later reaction times are perfect representations of the reaction mixture at any given instant in time. In situ spectroscopic monitoring would be helpful but is complicated by strong interferences in the NIR by the solvents and surfactants used to synthesize these particles.
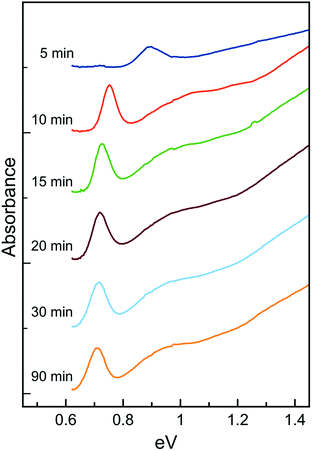 | ||
| Fig. 3 NIR spectra from PbS NCs collected during the course of a reaction. Particle growth is rapid during the first few minutes, but then slows considerably as the precursors are depleted. | ||
NCs prepared by this method can attain narrow size distributions. Fig. 4 shows transmission electron microscopy (TEM) images of PbS NCs that were reacted for 45 minutes. These NCs exhibit a mean particle size of 8.4 nm, and the distribution has a relative standard deviation of 0.75 nm (9% r.s.d.).
Aside from the induction time mentioned earlier, the growth trajectory of these NCs resembles those of many other NCs. Fig. 5 shows (a) a plot of exciton peak energy and (b) spectral peak width during the NC growth phase. These data were obtained from a series of spectra similar to those depicted in Fig. 3. Multiple Gaussian peaks were fit to the spectra to obtain reliable values for the spectral position and width of the lowest energy exciton peak. The data show that particle size grows rapidly over the first 15–20 minutes while significant quantities of precursors remain in the solution. The particle growth is reflected in a rapid red shift of the exciton peak. After about 20 minutes, the NC precursors are depleted and particle growth slows considerably. The early rapid growth phase is accompanied by significant focusing (narrowing) of the particle size distribution, as indicated by the spectral peak width (full-width-half-maximum) measurements shown in (b). Once the precursors are depleted and growth stalls, the particle size distribution stops narrowing and begins to broaden (defocus) very slowly through Ostwald ripening. Similar size focusing/defocusing has been previously observed for other NC systems and explained in relation to the Gibbs–Thomson effect.19
An unusual feature of this method is the inclusion of four different solvents, dimethylformamide (DMF), diphenyl ether (DPE), trioctylphosphine (TOP), and octadecene (ODE), in the reaction mixture. Of these, the ODE in the round bottom flask (used to prepare and dissolve the lead oleate) can be replaced with DPE without significantly affecting the course of the reaction. ODE is preferred over DPE here because it has lower vapor pressure, less odor, is easier to handle (does not freeze near room temperature), and is slightly cheaper. The other three solvents each have an important role in the reaction.
DMF is needed to quickly and completely dissolve the TAA.
The addition of TOP to both the syringe mixture and the round bottom flask improves the particle size distribution and also facilitates the purification process. If TOP is omitted, particle flocculation with alcohols becomes more difficult, and the particles do not re-disperse in solvents as readily following purification. The action of TOP in this reaction is similar to that described by Moreels et al. who observed that TOP changed the NC growth trajectory but did not appear to attach to the surface of the NC products. In the present case, TOP does modify the NC growth trajectory, but in spite of the differences in purification, it does not appear that TOP remains bound to the NC surface after purification. This conclusion is supported by FTIR measurements on our PbS NCs (ESI, Fig. S1†) if DPE is substituted for ODE in the Pb precursor mixture as mentioned above, then the addition of TOP into that mixture becomes essential because it inhibits the evaporation of DPE in accordance with Raoult's law. Without TOP added, the DPE evaporates during the initial evaluation at 95 °C and then re-condenses as a solid near the bottom of the condenser. This tendency is a significant drawback to using DPE instead of ODE as the primary solvent.
Regardless of whether ODE or DPE is chosen as solvent for the Pb precursor, DPE is essential in the injection solution containing the S precursor. The role of DPE in this mixture is to promote mixing of the DMF/TAA solution with the added TOP. In the absence of DPE, DMF and TOP form separate phases. In order to form a single phase, the DPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
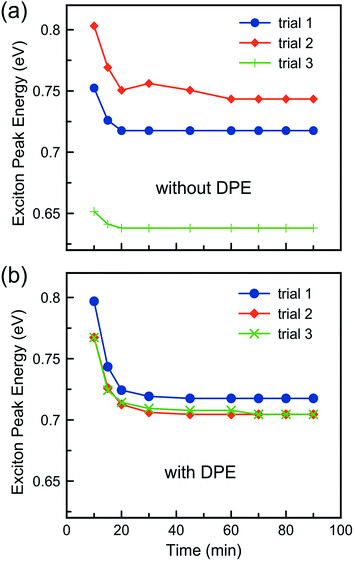
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOP ratio must fall within a certain range. While the phase separation is not entirely detrimental to the reaction, it does affect the particle growth trajectory and also the batch-to-batch particle size reproducibility as shown in Fig. 6.
TOP ratio must fall within a certain range. While the phase separation is not entirely detrimental to the reaction, it does affect the particle growth trajectory and also the batch-to-batch particle size reproducibility as shown in Fig. 6.
Fig. 6 shows the exciton peak energy for the final product obtained from various batches of PbS NCs prepared (a) without DPE added to the TAA injection mixture and (b) with DPE added. The addition of DPE significantly improves the reproducibility of the reaction, reducing the batch-to-batch relative standard deviation of the mean particle diameter from 17% down to 2%. The figure reveals that the variability in the absence of DPE originates in the early stages of the reaction with the nucleation event. DPE promotes homogeneous dispersion of the TAA throughout the reaction mixture so that nucleation is well-controlled and consistent. In the absence of DPE, the TAA is inhomogeneously distributed, leading to poor reproducibility in the nucleation event.
When DPE is omitted from the injection, TEM and spectral data reveal that numerous small nuclei are formed after injection, and these small “seeds” serve as a source of reactive monomers as the main population of larger particles progress to their final size. Fig. 7 shows a TEM image of a sample prepared without DPE in the injection. The main particle size distribution is still quite good, but there are a number of tiny nuclei present in this sample, which was reacted for 45 minutes. The small nuclei give rise to additional, short-wavelength peaks in both the absorbance and fluorescence spectra of the sample. As the reaction proceeds, the number of these nuclei decreases, and after about 90 minutes, they are almost entirely gone from the mixture.
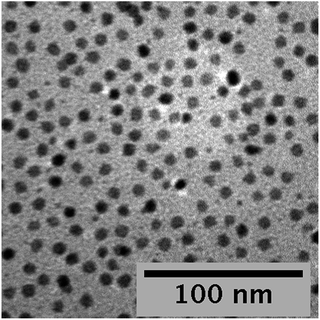 | ||
| Fig. 7 TEM image of PbS NCs prepared from lead oleate and TAA. No DPE was included in the TAA injection mixture, and small PbS nuclei can be detected in the sample. | ||
A particular advantage of the method described here is the simple and effective purification. The NCs can be completely separated from excess precursors, solvent, and reaction byproducts by simply mixing with chloroform and ethanol followed by centrifugation. Typically, a small volume of the NC reaction mixture is collected and mixed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NCs
1 (NCs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol
ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform), and this mixture is vortexed momentarily before centrifuging at high speed for 1–3 minutes. The NCs are collected as a solid and the clear, colorless supernatant is discarded. This process is quite effective, but to achieve even higher purity, the process can be repeated by re-suspending the NCs in a small volume of chloroform and then adding ethanol dropwise until turbid. After centrifuging, the clear, colorless supernatant can be discarded and the NCs can be dried under a stream of dry N2 to promote evaporation of CHCl3 and ethanol. Effectiveness of the purification can be assessed by monitoring the NIR absorption spectrum for organic impurities. Alkyl and aryl groups tend to give rise to narrow peaks between 1700 and 1800 nm, and residual alcohol produces a sharp peak near 1400 nm. Pure, OlAc-capped PbS NCs can be obtained within minutes after the reaction is finished.
chloroform), and this mixture is vortexed momentarily before centrifuging at high speed for 1–3 minutes. The NCs are collected as a solid and the clear, colorless supernatant is discarded. This process is quite effective, but to achieve even higher purity, the process can be repeated by re-suspending the NCs in a small volume of chloroform and then adding ethanol dropwise until turbid. After centrifuging, the clear, colorless supernatant can be discarded and the NCs can be dried under a stream of dry N2 to promote evaporation of CHCl3 and ethanol. Effectiveness of the purification can be assessed by monitoring the NIR absorption spectrum for organic impurities. Alkyl and aryl groups tend to give rise to narrow peaks between 1700 and 1800 nm, and residual alcohol produces a sharp peak near 1400 nm. Pure, OlAc-capped PbS NCs can be obtained within minutes after the reaction is finished.
The purification process does not involve size selection, as evidenced by the clear, colorless supernatant. This contrasts with other recently-reported methods that boast extremely narrow size distributions.4 As mentioned above, the present method routinely provides exciton spectral peak widths of 60 meV or less, with <10% relative standard deviation in the size distribution.
PbS NCs prepared by this method are stable against changes in size distribution when stored in their crude reaction mixture. Samples stored for periods of up to one month in this way have been re-suspended in TCE with no noticeable changes in NIR absorbance.
The results reported here have been obtained using ≥99.9% PbO and technical grade (90%) HOlAc to produce Pb(Ol)2. Although this synthesis has also been tested using high purity (99.999%) PbO and using Hendricks's method for producing anhydrous Pb(OlAc)2,6 those modifications did not noticeably improve sample quality, size distribution, stability, or photoluminescence quantum yield (PLQY). Indeed, we have found that the specific source of PbO and TAA can influence the final size distribution of the particles obtained. When supplier or reagent purity grade was changed, we typically saw changes in the overall mean size—and sometimes in the monodispersity—of the product particles.
Size tuning
Mean particle size can be tuned by altering the amount of excess HOlAc introduced into the system or by simply diluting the reaction through the addition of a non-coordinating solvent (ODE). Increasing the amount of excess HOlAc (beyond the minimum amount required to react with PbO) suppresses nucleation and early growth and leads to slightly larger particles. Diluting the reaction with non-coordinating solvent has the opposite effect, presumably through the dilution of surface capping ligands such as HOlAc, OlAc−, and TOP. These trends are identical to the behavior that was originally reported by Yu et al. for CdS.22The tendency for excess HOlAc to suppress nucleation and early particle growth is evident from observations on the early reaction kinetics. In particular, we have observed that the induction period that precedes darkening of the reaction mixture varies significantly as HOlAc is increased (Table 1). As the induction period increased in these experiments, the particle size was also observed to increase slightly, while reaction yield remained almost constant. The increasing particle size at higher [HOlAc] under conditions of invariant yield implies that fewer nuclei are formed in the presence of higher [HOlAc]. Reducing the amount of excess HOlAc allows spectral tuning of the first exciton peak down to about 1700 nm. Increasing the amount of excess HOlAc permits tuning to larger size and longer wavelengths, but as the wavelength shifts beyond 1900 nm, the peak width broadens significantly due to size inhomogeneity. We find that another method makes it possible to obtain large particles without sacrificing monodispersity, as explained below.
OlAc−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb2+ mole ratio Pb2+ mole ratio |
Induction time (s) | First exciton wavelength (nm) |
|---|---|---|
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1** 1** |
≤40 | 1700 |
2.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
155 | 1716 |
3.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
200 | 1883 |
Lowering the concentration of both the Pb and S precursors leads to a further reduction in final NC size. The dilution is most easily achieved by simply increasing the amount of solvent (ODE) in the reaction. For example, the particles obtained using 5 mL ODE, 1.5 mmol PbO, and 5.5 mmol HOlAc are typically in the 8.0 nm range, with an exciton absorption peak near 1800 nm (0.70 eV). If the amount of ODE included in the reaction is increased six-fold to 30 mL, the mean particle size decreases to approximately 5.5 nm, with an exciton absorption peak near 1500 nm (0.83 eV). This increase in ODE corresponds to approximately tripling of the total reaction volume, from almost 15 mL to almost 40 mL. By combining dilution with reduction in the amount of excess HOlAc, it is possible to tune the exciton peak position to wavelengths shorter than 1400 nm, corresponding to NCs smaller than 5 nm in diameter (Fig. 8). We have found that this same means of size tuning also applies when preparing PbS NCs by the Hendricks method, using thiourea precursors.6
Particles larger than those obtained by the standard method can be obtained through the use of multiple precursor injections as has been described by others for other NC systems.19,23 Gradual injection of TAA via syringe pump allows further NC growth with this system. Since the original system has a three-fold excess of Pb, additional Pb is not necessary to grow the NCs further unless very large NCs are desired. Fig. 9(a) shows near infrared (NIR) absorption spectra taken from NC samples during the course of a second injection of TAA. The solid curve shows the spectrum of the sample 70 minutes after the first injection, but prior to the start of the second injection. The sample was kept at 85 °C during the 70 minutes initial reaction, but particle growth had halted, and the spectrum was nearly unchanged for over 30 minutes of that time. Once the second TAA injection began, the exciton peak began to red shift again, indicating renewed NC growth. The dashed and dotted line spectra in Fig. 9(a) were taken from samples collected at ten minute intervals during the second TAA injection, which lasted 40 minutes. The amount of TAA injected was the same as the first injection, so the total amount of PbS in the system at the end of the second injection was exactly twice the amount synthesized in the first injection. The 40 minutes injection duration was selected to roughly correspond to the rate of precursor consumption and incorporation into the NCs after the initial injection. From the exciton peak wavelength, the dominant NC diameter can be calculated for each spectrum (Fig. 9(b)) in accordance with Cademartiri's formula.24 From these values, the fractional NC volume change can be estimated as a function of the amount of TAA injected into the system (Fig. 9(c)). The data show that the incorporation of PbS into the existing NCs is linear and that the NC size almost exactly doubles by the time the second TAA injection is complete. These results are consistent with quantitative incorporation of injected sulfur into the existing NCs with negligible new nucleation. TEM images taken after the second injection are also consistent with this picture, as the particle size distribution remains good after multiple injection growth.
Quantum yield
Preparation of smaller NCs allowed for measurement of PL emission intensity using an InGaAs detector. Fig. 8 showed absorbance and uncorrected PL emission spectrum from PbS NCs. A 980 nm diode laser was used for excitation. The as-prepared 5.5 nm NCs were luminescent, and the PL spectrum shows only a single peak, confirming the absence of smaller particles in the sample. The quantum yield of the smaller NC samples we prepare typically show quantum yield (PLQY) of 25 ± 10%. This quantity has a large estimated uncertainty because the NC luminescence is spectrally far removed from that of our quantum yield standard (IR-26), and the PL emission from all NC samples tested were near the long wavelength cutoff of the detector. Our measured PLQY is somewhat lower than the very best PLQY values that have been reported for PbS NCs, but no ligand exchange or further workup was carried out on these samples.4 In contrast, the Weidman approach requires significant post-preparative work-up and ligand exchange, and we expect that improved control over surface chemistry in our NCs—either through synthetic refinements or post-preparative handling—could provide further increases in PLQY.Conclusions
New information concerning the synthesis of PbS nanocrystals (NCs) from thiocarbonyl precursors has been presented, with special emphasis on thioacetamide (TAA). These reactions provide a simple and reproducible route to high quality PbS NCs. The reagents used are stable and easy to handle, and the particle purification is fast and easy. The samples show good monodispersity (<10% r.s.d.) without the need for any size selective procedures, and particle size can be tuned across a wide range either by controlling concentration or by employing multiple precursor injection steps. The particles show good photoluminescence quantum yields in the NIR without the addition of a protective shell.Acknowledgements
This material is based on work supported by the National Science Foundation under Grant No. CHE-1307847 and EPS-1004083. WRT acknowledges support from the MTSU Undergraduate Research & Creative Activity (URECA) program.Notes and references
- M. A. Hines and G. D. Scholes, Adv. Mater., 2003, 15, 1844 CrossRef CAS.
- L. Cademartiri, J. Bertolotti, R. Sapienza, D. S. Wiersma, G. von Freymann and G. A. Ozin, J. Phys. Chem. B, 2006, 110, 671 CrossRef CAS PubMed.
- I. Moreels, Y. Justo, B. De Geyter, K. Haustraete, J. C. Martins and Z. Hens, ACS Nano, 2011, 5, 2004 CrossRef CAS PubMed.
- M. C. Weidman, M. E. Beck, R. S. Hoffman, F. Prins and W. A. Tisdale, ACS Nano, 2014, 8, 6363 CrossRef CAS PubMed.
- W. R. Tilluck and P. G. Van Patten, to be published.
- M. P. Hendricks, M. P. Campos, G. T. Cleveland, I. Jen-La Plante and J. S. Owen, Science, 2015, 348, 1226 CrossRef CAS PubMed.
- D. Zherebetskyy, M. Scheele, Y. Zhang, N. Bronstein, C. Thompson, D. Britt, M. Salmeron, P. Alivisatos and L. W. Wang, Science, 2014, 344, 1380 CrossRef CAS PubMed.
- A. Lobo, T. Möller, M. Nagel, H. Borchert, S. G. Hickey and H. Weller, J. Phys. Chem. B, 2005, 109, 17422 CrossRef CAS PubMed.
- M. Nagel, Größen- und formselektive Synthese von PbS Nanopartikeln und deren Kristallisation in 2D und 3D Übergittern, PhD Dissertation, Universität Hamburg, Hamburg, Germany, 2007.
- C. Schliehe, B. H. Juarez, M. Pelletier, S. Jander, D. Greshnykh, M. Nagel, A. Meyer, S. Foerster, A. Kornowski, C. Klinke and H. Weller, Science, 2010, 329, 550 CrossRef CAS PubMed.
- N. A. Dhas, A. Zaban and A. Gedanken, Chem. Mater., 1999, 11, 806 CrossRef CAS.
- W.-S. Chae, H.-W. Shin, E.-S. Lee, E.-J. Shin, J.-S. Jung and Y.-R. Kim, J. Phys. Chem. B, 2005, 109, 6204 CrossRef CAS PubMed.
- T. Rath, B. Kunert, R. Resel, G. Fritz-Popovski, R. Saf and G. Trimmel, Inorg. Chem., 2008, 47, 3014 CrossRef CAS PubMed.
- E. D. Spoerke, M. T. Lloyd, Y.-J. Lee, T. N. Lambert, B. B. McKenzie, Y.-B. Jiang, D. C. Olson, T. L. Sounart, J. W. P. Hsu and J. A. Voigt, J. Phys. Chem. C, 2009, 113, 16329 CAS.
- A. de Kergommeaux, M. Lopez-Haro, S. Pouget, J. M. Zuo, C. Lebrun, F. Chandezon, D. Aldakoy and P. Reiss, J. Am. Chem. Soc., 2015, 137, 9943 CrossRef CAS PubMed.
- J. N. Demas and G. A. Crosby, J. Phys. Chem., 1971, 75, 991 CrossRef.
- O. E. Semonin, J. C. Johnson, J. M. Luther, A. G. Midgett, A. J. Nozik and M. C. Beard, J. Phys. Chem. Lett., 2010, 1, 2445 CrossRef CAS.
- S. Hatami, C. Wurth, M. Kaiser, S. Leubner, S. Gabriel, L. Bahrig, V. Lesnyak, J. Pauli, N. Gaponik, A. Eychmuller and U. Resch-Genger, Nanoscale, 2015, 7, 133 RSC.
- X. G. Peng, J. Wickham and A. P. Alivisatos, J. Am. Chem. Soc., 1998, 120, 5343 CrossRef CAS.
- C. B. Murray, C. R. Kagan and M. G. Bawendi, Annu. Rev. Mater. Sci., 2000, 30, 545 CrossRef CAS.
- V. K. LaMer and R. H. Dinegar, J. Am. Chem. Soc., 1950, 72, 4847 CrossRef CAS.
- M. W. Yu and X. G. Peng, Angew. Chem., Int. Ed., 2002, 41, 2368 CrossRef.
- W. W. Yu, Y. A. Wang and X. G. Peng, Chem. Mater., 2003, 15, 4300 CrossRef CAS.
- L. Cademartiri, E. Montanari, G. Calestani, A. Migliori, A. Guagliardi and G. A. Ozin, J. Am. Chem. Soc., 2006, 128, 10337 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12592a |
| This journal is © The Royal Society of Chemistry 2016 |