Immobilization of Ophiopogonin D on stainless steel surfaces for improving surface endothelialization†
Kun Zhangacd,
Xiaofeng Wangc,
Fangxia Guana,
Qian Lic and
Jingan Li*bcd
aSchool of Life Science, Zhengzhou University, 100 Science Road, Zhengzhou 450001, PR China
bSchool of Material Science and Engineering, Zhengzhou University, 100 Science Road, Zhengzhou 450001, PR China. E-mail: 4828713@qq.com; Tel: +86-13551284797
cNational Center for International Research of Micro-nano Molding Technology, Key Laboratory for Micro Molding Technology of Henan Province, 100 Science Road, Zhengzhou 450001, PR China
dKey Lab. for Advanced Technologies of Materials, Ministry of Education, School of Material Science and Engineering, Southwest Jiaotong University, Chengdu 610031, PR China
First published on 24th November 2016
Abstract
Ophiopogonin D, a traditional Chinese medicinal ingredient, was immobilized to form a coating onto the surface of 316L stainless steel (316L SS), which is often used in cardiovascular implant materials, and evaluated for its endothelialization ability in vitro. The immobilized coating showed more hydrophilic property compared with the 316L SS control, contributing to protein absorption and cell spreading. A CCK-8 assay was performed to investigate the attachment and proliferation of vascular endothelial cells (VEC) onto the coating, and the results showed that there were more VEC on the Ophiopogonin D coating than on the 316L SS surface. The acridine orange (AO)/propidium iodide (PI) staining images of the VEC proved that the Ophiopogonin D coating could effectively inhibit VEC apoptosis, compared with the control. In addition, VEC on the Ophiopogonin D coating released more nitric oxide (NO) and PGI2 compared with VEC on the 316L SS. All the results indicated that Ophiopogonin D could significantly improve surface endothelialization and possessed potential applications for the surface modification of cardiovascular devices.
1. Introduction
Cardiovascular disease is a class of diseases that usually results from malfunction in coronary arteries and has been the leading cause of morbidity and mortality globally for many years.1–3 The main causes of cardiovascular disease may be attributed to atherosclerosis and thrombosis, which lead to vascular luminal narrowing and occlusion.4,5 Currently, vascular intervention using a stent is an effective way of treating these diseases.6,7 However, despite all the immediate benefits, the stent implantation also provokes injury-induced inflammation, hyperplasia and subsequent in-stent restenosis (ISR).8 The application of drug-eluting stents (DES) has been reported to effectively decrease the incidence of ISR by 50–70%.8 Thus far, various anticoagulant and/or antihyperplastic drugs, such as rapamycin and paclitaxel, have been used for DES to inhibit ISR.9,10 Nevertheless, the drugs loaded on DES delayed vascular healing and re-endothelialization, which led to high risk of late hyperplasia and thrombosis.8Vascular endothelial cells (VEC) play crucial roles in maintaining vascular homeostasis.11 First, the primary physiological function of VEC is to provide an appropriate blood compatible surface to guarantee blood flow.12 In addition, healthy endothelium effectively controls smooth muscle cell proliferation.8 Most of these physiological processes are performed via the functional factors released from VEC, such as nitric oxide (NO) and prostacyclin (PGI2).13,14 Therefore, an endothelial-friendly surface that not only increases VEC proliferation, but also inhibits VEC apoptosis and promotes its functional factor release is very important to reduce thrombosis and restenosis of a stent.
Ophiopogonin D is the main ingredient of several clinical injections and oral drugs applied for the treatment of cardiovascular disease for the protection of VEC.15,16 It has been proven by Qian et al. that Ophiopogonin D can reduce VEC apoptosis by stabilizing the mitochondrial membrane potential and reducing cell permeability.15 Several studies even demonstrate that Ophiopogonin can improve NO and PGI2 release from the VEC,17,18 although it is still uncertain which Ophiopogonin plays the key role. Ophiopogonin D can only be isolated from a traditional Chinese medicine called Ophiopogon japonicas, which grows all over the world as green vegetation, but it has been used as a drug in China for more than two thousand years (Fig. 1A and B); it was earliest described in Shennong Herbal Classic, which was created in the Qin dynasty. In the past decades, several injections or oral drugs for cardiovascular diseases have been prepared onto the implanted device surface.19–21 However, to date, little has been reported on Ophiopogonin D being applied in the surface modification of a cardiovascular implanted device. Therefore, in the present study, it was chosen to fabricate a medical coating for the biomaterial surface that would promote endothelialization.
 | ||
| Fig. 1 (A) Plants and (B) crude drug of Ophiopogon japonicas; (C) chemical structure of Ophiopogonin D. | ||
The aim of this study was to develop an Ophiopogonin D-immobilized surface and investigate the effect on endothelial attachment, proliferation, viability and functional factor release. Thus, we prepared an Ophiopogonin D coating on the 316L stainless steel (316L SS) surface via chelation reaction. A toluidine blue O (TBO) assay was performed to detect the quantity of immobilized Ophiopogonin D, and the morphology and roughness of the coating was characterized by atomic force microscopy (AFM). The wettability of the Ophiopogonin D coating was measured by a water contact angle apparatus. We hope this research will contribute to the further study and application of Ophiopogonin D to the surface modification of cardiovascular implanted devices.
2. Materials and methods
2.1 Fabrication of the Ophiopogonin D coating
The Ophiopogonin D coating was fabricated on the 316L stainless steel (316L SS) substrates via a deposition method: briefly, the 316L SS substrates (diameter = 10 mm, Baoji, China) were polished and cleaned ultrasonically three times with acetone, ethanol, and deionized water (dH2O), successively, and then dried at room temperature (RT). Next, the 316L SS substrates were immersed into a Ophiopogonin D (powder, analytic grade, Dikang, China) solution with an initial concentration of 2.0 mg ml−1 (diluted in 10 mM Tris buffer, pH 8.5) at 37 °C for 24 h and then cleaned ultrasonically three times with dH2O, and dried at RT. Because there are lots of ortho-hydroxy (o-hydroxy) groups in the Ophiopogonin D molecule (Fig. 1C), it could be immobilized on the 316L SS surface by chelation. The sample was briefly labeled as OPH, and the entire fabrication process is presented in Fig. 2.2.2 Characterization of the Ophiopogonin D coating
To ensure that the Ophiopogonin D coating was successfully immobilized onto the 316L SS substrate, the quantitative characterization was performed with the toluidine blue O (TBO) assay as described by Li et al.22 In brief, the samples immobilized with OPH were incubated in 5 ml of a freshly prepared solution of 0.04 wt% TBO in aqueous 0.01 M HCl/0.2 wt% NaCl. Then, the samples were gently shaken at 37 °C for 4 h and rinsed twice with dH2O; during this process, an OPH/TBO complex was formed on the sample surface. Following this, 5 ml of a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of ethanol and 0.1 M aqueous NaOH were added. Furthermore, the OPH/TBO complex was dissolved and released into the liquid phase. After complete dissolution of the complex, 200 μl of the supernatant was added to a 96-well plate, and the OD value was obtained with a microplate reader at 530 nm. The OD value was used to calculate the amount of immobilized OPH from a calibration curve. The surface morphology of the OPH coating on the 316L SS substrate was observed using optical microscopy (Axio Lab.A1, ZEISS, GERMANY), and its roughness was characterized by atomic force microscopy (AFM, JPK Instruments, Berlin, Germany) in tapping mode with an scanning range of 400 nm × 400 nm.23 To evaluate the hydrophilicity of the OPH coating, the water contact angles (WCA) of the OPH and 316L SS samples were measured with a contact angle apparatus (DSA 100, Krüss, GmbH, Germany). The dried samples were fixed to an object stage, and a droplet of dH2O was added onto the surface to measure the contact angle (N = 6).24
1 (v/v) mixture of ethanol and 0.1 M aqueous NaOH were added. Furthermore, the OPH/TBO complex was dissolved and released into the liquid phase. After complete dissolution of the complex, 200 μl of the supernatant was added to a 96-well plate, and the OD value was obtained with a microplate reader at 530 nm. The OD value was used to calculate the amount of immobilized OPH from a calibration curve. The surface morphology of the OPH coating on the 316L SS substrate was observed using optical microscopy (Axio Lab.A1, ZEISS, GERMANY), and its roughness was characterized by atomic force microscopy (AFM, JPK Instruments, Berlin, Germany) in tapping mode with an scanning range of 400 nm × 400 nm.23 To evaluate the hydrophilicity of the OPH coating, the water contact angles (WCA) of the OPH and 316L SS samples were measured with a contact angle apparatus (DSA 100, Krüss, GmbH, Germany). The dried samples were fixed to an object stage, and a droplet of dH2O was added onto the surface to measure the contact angle (N = 6).24
2.3 VEC culture
Vascular endothelial cells (VEC) were obtained from a newborn umbilical cord via a typical method.25,26 Briefly, the umbilical cord was cannulated and washed thoroughly with PBS to remove the blood inside the lumen. Then, 0.1% type II collagenase (Gibco BRL, USA) in M199 was introduced and incubated at 37 °C for 10 min. The digested cells were washed in serum-free medium and separated from the supernatant by centrifugation at 1200 rpm, and then collected in complete M199 containing 15% fetal calf serum, 50 mg ml−1 EC growth factor (ECGS, Sigma), 100 mg ml−1 heparin, 20 mmol l−1 HEPES, and 2 mmol l−1 L-Gln. The suspended cells were then seeded in a cell culture flask and incubated at 37 °C in an atmosphere containing 5% CO2. Replicated cultures were performed by trypsinization when cells were approaching confluence. Cells were fed with fresh complete M199 every 48 h. VEC between passages 3 and 5 were used for experiments to ensure the genetic stability of the cultures. The OPH and 316L SS samples were placed in a 24-well culture plate, and VEC were seeded onto the samples at a concentration of 5 × 104 cells per ml, then cultured at 37 °C for 1 and 3 days, respectively. A CCK-8 assay was performed to investigate the VEC attachment and proliferation on the samples.27To study the viability of VEC on the samples, cell apoptosis was detected using fluorescence staining with the acridine orange (AO)/propidium iodide (PI) kit (Boshide, China).28 In detail, VEC were seeded onto the samples at a density of 5 × 104 cells per ml for 1 and 3 days. Subsequently, the cells were stained with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of AO (100 mg ml−1) and PI (100 mg ml−1) at 37 °C for 5 min, and then immediately inspected under a fluorescence microscope. Green cells were vital, whereas orange cells were apoptotic.
1 mixture of AO (100 mg ml−1) and PI (100 mg ml−1) at 37 °C for 5 min, and then immediately inspected under a fluorescence microscope. Green cells were vital, whereas orange cells were apoptotic.
The nitric oxide (NO) release from the VEC was determined using the Griess Reagent method,29 and the prostacyclin (PGI2) secreted by the VEC was determined by related kits according to the manual after 1 day and 3 days culture, respectively.30 Both functional factors were normalized to the cell number.
The newborn umbilical cords used for the VEC experimental protocols were approved by the Maternal and Child Health Hospital Institutional Review Board (Chengdu, China). Our research complied with the Helsinki Declaration. All experiments were performed in compliance with the guidelines of “Biomedical research ethics review method involving people” (China), and approved by the medical ethics committee at Maternal and Child Health Hospital in Chengdu, China. Informed consents were obtained from human participants of this study.
2.4 Statistical analysis
The data were statistically evaluated using ANOVA by homogeneity test of variances. Data were expressed as mean ± standard deviation (SD). A probability value of p < 0.05 was considered as a significant difference. The data analysis was performed using the software SPSS 11.5 (Chicago, IL).3. Results and discussion
3.1 Characterization of Ophiopogonin D coating
The amount of Ophiopogonin D immobilized was determined by the TBO method at room temperature. Fig. S1† shows the amount of Ophiopogonin D bound on the OPH sample, and 316L SS was used as a control. The results showed that there was 3.59 ± 0.34 μg cm−2 of Ophiopogonin D immobilized on the OPH sample, suggesting a successful preparation of the Ophiopogonin D coating on the 316L SS surface.The surface morphologies of the OPH coating and the 316L SS substrate were observed using optical microscopy, and the results are displayed in Fig. S2.† The 316L SS substrate showed a smooth and clear surface under the optical microscopy, whereas after the Ophiopogonin D immobilization, a rough coating on the surface was clearly visible. The roughness of the OPH coating and the 316L SS substrate were characterized by AFM and presented in Fig. 3A. It is clear that the roughness of the 316L SS surface increased from 3.3 ± 0.6 nm to 19.0 ± 3.1 nm after coating with Ophiopogonin D. The OPH surface seemed rougher as compared with the 316L SS surface, and there were several particles or tips with nanoscale distribution on the OPH surface. These nanoscale structures may have contributed to cell adhesion and proliferation.31,32
 | ||
| Fig. 3 (A) AFM images and (B) water contact angle of the OPH and 316L SS surfaces (*p < 0.05 compared with 316L SS, mean ± SD, n = 6). | ||
The water contact angle was measured as a function of the 316L SS substrate processing (Fig. 3B). Compared with 316L SS, the water contact angle dramatically decreased to 20.3° ± 6.9° after immobilized with Ophiopogonin D, indicating more hydrophilicity as compared to the 316L SS substrate, and this property may have contributed to the protein absorption and cell attachment.33
3.2 VEC viability and coagulant factor release
Generally, mammalian cells undergo a cell adhesion process of substrate attachment, spreading, cytoskeleton development, proliferation and gradual apoptosis, simultaneously.28 To investigate the attachment and proliferation of VEC on the OPH coating, VEC obtained from human umbilical cords were seeded on its surface, and the 316L SS substrate was used as a control. After incubation for 1 and 3 days, the quantity of VEC was determined by the CCK-8 assay. Fig. 4 shows the amount of VEC on the 316L SS and on the surface coated with Ophiopogonin D. It can be seen that after incubating for 1 day, there is a higher VEC count on the Ophiopogonin D immobilized surface than on the pristine 316L SS surface. The reason may be that the hydrophilic surface of the OPH coating can absorb more protein and/or growth factors from the full-component medium as compared with the pristine 316L SS surface, which contributes to the attachment of the VEC.34,35 After a 3 day culture, the OPH coating still contained a higher amount of VEC with respect to the 316L SS surface, suggesting a better ability to promote VEC proliferation. It was reported by Liu et al. that the micro- and/or nanoparticles possess an excellent property to improve VEC proliferation.36 | ||
| Fig. 4 Investigation of VEC amount on the OPH coating and the 316L SS using the CCK-8 assay after 1 day and 3 day incubation, respectively. (*p < 0.05 compared with 316L SS, mean ± SD, n = 3). | ||
Fig. 5 shows the fluorescence microscopy images of the apoptotic or necrotic VEC (in red). On the 316L SS surface, VEC showed numerous apparent apoptotic vesicles surrounding them, which indicated serious apoptosis or necrosis, but the VEC on the OPH coating showed no apoptosis. The statistical results are displayed in Fig. 6. It can be seen that the OPH coating showed less apoptotic or necrotic VEC compared with the pristine 316L SS, suggesting that there were more vital cells than those on the 316L SS surface. The results indicated that VEC could have a better viability on the OPH coating than on the pristine 316L SS surface during a 3 day culture.15,16
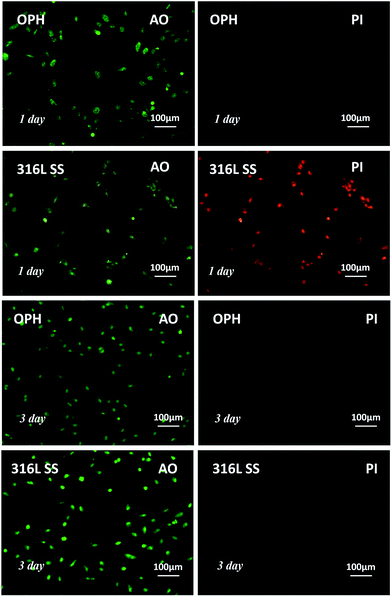 | ||
| Fig. 5 VEC proliferation and viability on the OPH coating and reference surface (green: vital cells, red: dead cells). | ||
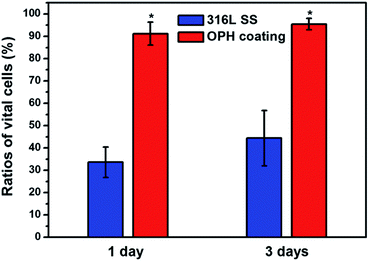 | ||
| Fig. 6 Calculation of ratios of the vital VEC on the OPH coating and reference surfaces (*p < 0.05 compared with other samples, mean ± SD, n = 3). | ||
Healthy VEC in native blood vessels can inhibit platelet activation and aggregation, regulate vasodilation and suppress smooth muscle cell proliferation by continuously producing nitric oxide (NO) and prostacyclin (PGI2).37,38 Therefore, the NO and PGI2 released by the VEC on the OPH coating and the pristine 316L SS surface were examined and is shown in Fig. 7. As it can be observed in the results, VEC on the OPH coating released more NO and PGI2 compared with the VEC on the pristine 316L SS surface, demonstrating a better anticoagulant function. This may be attributed to the lower apoptosis rate of VEC on the OPH coating; the OPH coating reduced the apoptosis ratio of VEC and increased the VEC activity, which contributed to the NO and PGI2 release from the VEC.39,40
 | ||
| Fig. 7 Detection of NO and PGI2 release from VEC on the OPH coating and the reference surface. (*p < 0.05 compared with other samples, mean ± SD, n = 3). | ||
4. Conclusions
In this study, the biocoating of a traditional Chinese drug (TCD), Ophiopogonin D, was successfully applied onto the 316L SS surface via chelation reaction for VEC attachment, proliferation, viability and anticoagulant factor release, simultaneously. Certainly, Ophiopogonin D still maintained its ability to protect VEC and inhibit VEC apoptosis. The hydrophilicity of the OPH coating induced an increased VEC attachment on the surface, and the nanostructure formed during the chelation process endowed the OPH coating with the ability of improving VEC proliferation. Our data also demonstrated that the OPH coating promoted NO and PGI2 release from VEC. We hope that this basic research on OPH coating will be helpful for more TCD surface development, and have potential applications for surface modification of cardiovascular implanted devices.Acknowledgements
This study was supported by the China Postdoctoral Science Foundation (2014M562333 and 2015M582206), the Joint Fund for Fostering Talents of National Natural Science Foundation of China and the Henan province (U1504310), the Joint Fund for Fostering Talents of NCIR-MMT & HNKL-MMT (MMT2016-09), the Natural Science Foundation of China (81471306), the International Science & Technology Cooperation Program of China (2015DFA30550), and the Key Project of International Cooperation of the Henan Province (152102410013).Notes and references
- S. E. Hyman, Sci. Transl. Med., 2014, 6, cm9 CrossRef PubMed.
- J. D. Berry, A. Dyer, X. Cai, D. B. Garside, H. Y. Ning, A. Thomas, P. Greenland, L. V. Horn, R. P. Tracy and D. M. Lloyd-Jones, N. Engl. J. Med., 2012, 366, 321 CrossRef CAS PubMed.
- L. X. Jiang, H. M. Krumholz, X. Li, J. Li and S. S. Hu, Lancet, 2015, 386, 1493 CrossRef.
- C. K. Glass and J. L. Witztum, Cell, 2001, 104, 503 CrossRef CAS PubMed.
- A. J. Li, Biomedicine & Preventive Nutrition, 2013, 3, 339 Search PubMed.
- T. P. Murphy, C. J. Cooper, A. H. Matsumoto, D. E. Cutlip, K. M. Pencina, K. Jamerson, K. R. Tuttle, J. I. Shapiro, R. D'Agostino, J. Massaro, W. Henrich and L. D. Dworkin, J. Am. Coll. Cardiol., 2015, 66, 2487 CrossRef PubMed.
- I. G. de Torre, F. Wolf, M. Santos, L. Rongen, M. Alonso, S. Jockenhoevel, J. C. Rodríguez-Cabello and P. Mela, Acta Biomater., 2015, 12, 146 CrossRef CAS PubMed.
- Y. Yang, P. K. Qi, F. Wen, X. Y. Li, Q. Xia, M. F. Maitz, Z. L. Yang, R. Shen, Q. F. Tu and N. Huang, ACS Appl. Mater. Interfaces, 2014, 6, 14608 CAS.
- P. Petrou and S. Dias, Int. J. Cardiol., 2016, 202, 448 CrossRef PubMed.
- S. Kufner, R. A. Byrne, A. de Waha, S. Schulz, M. Joner, K. L. Laugwitz and A. Kastrati, Cardiovascular Revascularization Medicine, 2014, 15, 69 CrossRef PubMed.
- F. Wu, J. A. Li, K. Zhang, Z. K. He, P. Yang, D. Zou and N. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 109 CAS.
- J. L. Chen, Q. L. Li, J. Q. Xu, L. Zhang, M. F. Maitz and J. Li, J. Mater. Chem. B, 2015, 3, 2615 RSC.
- M. Zhou, Y. Q. Bao, H. B. Li, Y. Pan, L. L. Shu, Z. Y. Xia, D. H. Wu, K. S. L. Lam, P. M. Vanhoutte, A. Xu, W. P. Jia and R. L. C. Hoo, Clin. Sci., 2015, 129, 547 CrossRef CAS PubMed.
- A. Borgognone, L. Navarro-Nunez, J. N. Correia, A. Y. Pollitt, S. G. Thomas, J. A. Eble, F. M. Pulcinelli, M. Madhani and S. P. Watson, J. Thromb. Haemostasis, 2014, 12, 550 CrossRef CAS PubMed.
- J. C. Qian, F. R. Jiang, B. Wang, Y. Yu, X. Zhang, Z. M. Yin and C. Liu, J. Ethnopharmacol., 2010, 128, 438 CrossRef CAS PubMed.
- C. H. Xia, G. J. Wang, J. G. Sun, H. P. Hao, Y. Q. Xiong, S. H. Gu, L. Shang and C. A. Zheng, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2008, 862, 72 CrossRef CAS PubMed.
- L. H. Li, J. S. Wang and L. Y. Kong, Chin. J. Nat. Med., 2013, 11, 222 CrossRef PubMed.
- S. L. Man, Y. L. Wang, Y. Y. Li, W. Y. Gao, X. X. Huang and C. Y. Ma, Chin. Herb. Med., 2013, 5, 33 Search PubMed.
- D. J. Kereiakes, R. W. Yeh, J. M. Massaro, P. Driscoll-Shempp, D. E. Cutlip, P. G. Steg, A. H. Gershlick, H. Darius, I. T. Meredith, J. Ormiston, J. F. Tanguay, S. Windecker, K. N. Garratt, D. E. Kandzari, D. P. Lee, D. I. Simon, A. C. Iancu, J. Trebacz and L. Mauri, JACC: Cardiovascular Interventions, 2015, 8, 1552 CrossRef PubMed.
- L. Yang, S. Whiteside, P. A. Cadieux and J. D. Denstedt, Asian Journal of Urology, 2015, 2, 194 CrossRef.
- R. Piccolo, T. Pilgrim, D. Heg, A. Franzone, J. Rat-Wirtzler, L. Räber, S. Silber, P. W. Serruys, P. Jüni and S. Windecker, JACC: Cardiovascular Interventions, 2015, 8, 1657 CrossRef PubMed.
- G. C. Li, P. Yang, Y. Z. Liao and N. Huang, Biomacromolecules, 2011, 12, 1155 CrossRef CAS PubMed.
- Y. Xu, J. A. Li, L. F. Yao, L. H. Li, P. Yang and N. Huang, Surf. Coat. Technol., 2015, 261, 436 CrossRef CAS.
- Z. Zhou, J. Chen, L. J. Xiang, Y. Xu, P. Yang, J. A. Li, J. J. Wu and N. Huang, Mater. Lett., 2014, 132, 149 CrossRef CAS.
- J. A. Li, K. Zhang, Y. Xu, J. Chen, P. Yang, Y. C. Zhao, A. S. Zhao and N. Huang, J. Biomed. Mater. Res., Part A, 2014, 102, 1950 CrossRef PubMed.
- J. A. Li, G. C. Li, K. Zhang, Y. Z. Liao, P. Yang, M. F. Maitz and N. Huang, Appl. Surf. Sci., 2013, 273, 24 CrossRef CAS.
- J. A. Li, K. Zhang, F. Wu, Z. K. He, P. Yang and N. Huang, J. Mater. Sci., 2015, 50, 3226 CrossRef CAS.
- J. A. Li, K. Zhang, H. Q. Chen, T. Liu, P. Yang, Y. C. Zhao and N. Huang, Mater. Sci. Eng., C, 2014, 38, 235 CrossRef CAS PubMed.
- J. A. Li, K. Zhang, P. Yang, W. Qin, G. C. Li, A. S. Zhao and N. Huang, Colloids Surf., B, 2013, 110, 199 CrossRef CAS PubMed.
- L. J. Xiang, J. A. Li, Z. K. He, J. J. Wu, P. Yang and N. Huang, Micro Nano Lett., 2015, 10, 287 Search PubMed.
- Y. Q. Wang and L. X. Chen, Nanomedicine, 2011, 7, 385 CAS.
- F. F. Du, H. Y. Zhou, L. X. Chen, B. Zhang and B. Yan, Trends Anal. Chem., 2009, 28, 88 CrossRef CAS.
- J. A. Li, P. Yang, K. Zhang, H. L. Ren and N. Huang, Nucl. Instrum. Methods Phys. Res., Sect. B, 2013, 307, 575 CrossRef CAS.
- L. J. Xiang, C. H. Li, P. Yang, J. A. Li and N. Huang, J. Eng., 2014, 7 DOI:10.1049/joe.2014.0274.
- J. A. Li, K. Zhang, P. Yang, L. L. Wu, J. L. Chen, A. S. Zhao, G. C. Li and N. Huang, Exp. Cell Res., 2013, 319, 2663 CrossRef CAS PubMed.
- T. Liu, Y. Liu, Y. Chen, S. H. Liu, M. F. Maitz, X. Wang, K. Zhang, J. Wang, Y. Wang, J. Y. Chen and N. Huang, Acta Biomater., 2014, 10, 1940 CrossRef CAS PubMed.
- L. H. Li, Y. Xu, Z. Zhou, J. Chen, P. Yang, Y. H. Yang, J. A. Li and N. Huang, Appl. Sci., 2015, 5, 1016 CrossRef.
- Q. Wen, K. O. Lee, S. Z. Sim, X. G. Xu and M. K. Sim, Eur. J. Pharmacol., 2015, 768, 173 CrossRef CAS PubMed.
- G. Csányi, M. Gajda, M. Franczyk-Zarow, R. Kostogrys, P. Gwoźdź, L. Mateuszuk, M. Sternak, L. Wojcik, T. Zalewska, M. Walski and S. Chlopicki, Prostaglandins Other Lipid Mediators, 2012, 98, 107 CrossRef PubMed.
- T. Mikkola, V. Ranta, A. Orpana, Y. Olavi and L. Viinikka, Maturitas, 1996, 25, 141 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17584h |
| This journal is © The Royal Society of Chemistry 2016 |

