Squaric acid derivative effects on the kinetics of photopolymerization of different monomers
Abstract
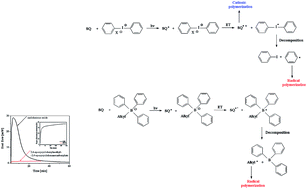
Systems composed of 1,3-bis(phenylamino)squaraine (photosensitizer) and conventional free radical sources, such as tetramethylammonium n-butyltriphenylborate, diphenyliodonium chloride and diphenyliodonium hexafluorophosphate were used for initiation of photopolymerization occurring via a radical or cationic mechanism. The photopolymerization of 1,6-hexanediol diacrylate (HDDA), pentaerythritol triacrylate (PETA), 2-ethyl-2-(hydroxymethyl)-1,3-propanediol triacrylate (TMPTA), cyclohexene oxide (CHO) and 3,4-epoxycyclohexylmethyl-3,4-epoxycyclohexanecarboxylate (EPOX) were carried out at 300 nm < λ < 500 nm irradiation. The polymerization kinetics was measured using a differential scanning calorimeter equipped with a high-pressure mercury lamp. The effect of co-initiator structure and type of monomer on the kinetics of the photopolymerization process is also presented here. It was found that the photoredox pairs, consisting of 1,3-bis(phenylamino)squaraine and tetramethylammonium n-butyltriphenylborate or diphenyliodonium salts, initiate radical and cationic polymerization in the UV-Vis light region. The photoinitiating ability of these new photoinitiating systems acting in the UV-Vis light region for initiation of polymerization of acrylates and epoxides was compared with a few commercially used photoinitiating systems.

 Please wait while we load your content...
Please wait while we load your content...