An efficient strategy for the synthesis of 5-hydroxymethylfurfural derivative based poly(β-thioether ester) via thiol-Michael addition polymerization†
Daihui Zhang,
Marie-Josée Dumont* and
Alice Cherestes
Department of Bioresource Engineering, McGill University, 21111 Lakeshore Rd., Sainte-Anne-de-Bellevue, QC, Canada. E-mail: marie-josee.dumont@mcgill.ca
First published on 30th August 2016
Abstract
A derivative of 5-hydroxymethylfurfural was synthesized for the thiol-Michael addition reaction. The efficiency of the catalysts (base and nucleophiles) and side reactions during the thiol-Michael addition were investigated. Dimethylphenylphosphine efficiently initiated the thiol-Michael addition polymerization for synthesizing bio-based furan polymers. The product poly(β-thioether ester) showed the potential to be functionalized.
The production of polymers from petroleum-based chemicals has caused serious sustainability concerns, thereby stimulating the development of bio-based polymers.1 5-Hydroxymethylfurfural (5-HMF) is a promising bio-based chemical which can be synthesized from several feedstocks having a rich hexose content.2 The functional groups of 5-HMF are available for derivatization3 which renders this platform chemical suitable for the synthesis of fine chemicals, biofuel precursors and monomers used in the synthesis of polymers.4 Techniques which have been used to synthesize polymers from 5-HMF derivatives include esterification or transesterification, enzymatic polymerization, ring opening polymerization and free radical polymerization.4d,5 However, these polymerization techniques are often performed under rigorous conditions where high temperature, vacuum and long reaction times are generally needed. Thus, new strategies requiring milder reaction conditions are desired.
The thiol-Michael addition reaction offers high conversion, mild reaction conditions and rapid reaction rate.6 The applications of thiol-Michael addition reaction range from small molecule synthesis, surface functionalization of materials and polymer modification, to networks formation.7 However, examples of the synthesis of linear polymers are few,8 especially involving the use of bio-based monomers. This could be due to side reactions occurring when phosphines and amine catalysts are used.8g,9 The presence of side-reactions is an issue when designing polymers through the step-growth polymerization technique since they can prevent the formation of the desired structure. The side-reactions also influence the molecular weight of linear polymers. Therefore, the elucidation of these side reactions is of importance in order to minimize their occurrence. This communication first reports on the efficiency of base and nucleophilic catalysts in addition to the potential side reactions occurring by the use of these catalysts. The second section of this communication focuses on the synthesis and characterization of bio-based furan polymers synthesized by the thiol-Michael addition polymerization. In addition, the incorporation of furan rings through 5-HMF derived monomers in the polymer chain enables the Diels–Alder reaction to occur.10 It can be used to tune the thermal and mechanical properties of materials. By this reaction, an organogel was synthesized in order to study the feasibility of the Diels–Alder reaction on furan rings.
The effects of three types of catalysts on the reaction rates, conversion, and potential side reactions were investigated using furfural acrylate and 1-propanethiol as the model substrates (Scheme 1). For the reaction, base catalysts (triethylamine (TEA) and 1,8-diazabicycloundec-7-ene (DBU)), nitrogen-centered nucleophiles (hexylamine and 4-dimethylamino-pyridine (DMAP)) and a phosphine-centered catalyst (dimethylphenyl-phosphine (DMPP)) were selected. The structure and molecular weights of furfural acrylate and Michael adduct were confirmed by 1H, 13C NMR and mass spectrometry, respectively (Fig. S1 and S2†). Table 1 shows the general reaction conditions and the effects of catalyst types on the thiol-Michael addition reaction. Typically, the base catalysed thiol-Michael reaction pathway involves the deprotonation of the thiol group to form a thiolate anion, as well as a conjugated acid.6 Then, the thiolate anion attacks the β-position of α,β-unsaturated carbonyl to yield a carbanion (strong base). The last step involves the abstraction of a proton from a conjugated acid by the carbanion to generate thioether as the product. For TEA, there was no conversion towards the desired Michael adduct (Table 1, entry 1). This was due to the similar pKa of the catalyst and 1-propanethiol. Thus, TEA failed to abstract a proton from 1-propanethiol. DBU, a non-nucleophile strong base, achieved over 99% conversion after 15 min, and the yield of the Michael adduct was >99% as determined by gas chromatography (GC) (entry 2). These results indicate that the choice of a suitable base catalyst can efficiently initiate the thiol-Michael addition of 1-propanethiol and furfural acrylate. However, it was noticed in previous studies that DBU functioned as a nucleophile as well.11 Therefore, DBU catalysed thiol-Michael addition is complex as it may involve a basic-nucleophilic hybrid mechanism.12 The potential mechanism of nucleophile-catalysed thiol-Michael addition is illustrated in Scheme 2A.13 The nucleophilic attack of the phosphine- or nitrogen-centered catalyst on the vinyl group generates the intermediate zwitterion. It can then deprotonate the thiol group to yield a thiolate anion. The formed thiolate anion further reacts with the vinyl group to generate the Michael adduct. Finally, the catalyst is recovered. Hexylamine serves as a good nucleophile catalyst for the thiol-Michael addition reaction.14 The conversion of furfural acrylate was >99% when using 2 mol% hexylamine catalyst and a reaction time of 8 h (entry 3) or when using 10 mol% catalyst and a reaction time of 4 h (entry 4). Chan et al. reported that the conversion of hexylamine catalysed reaction (0.096 mol%) of hexanethiol and hexyl acrylate was over 99% after 3 h at room temperature.12a Based on Li et al., the choice of the solvent allows the stabilization of the nucleophilic ion.9 There was 67% conversion of furfural acrylate after 24 h in chloroform (2 mol%, entry 5). It was suggested that the polarity of the solvent strongly impacted the stabilization of thiolate anions.9
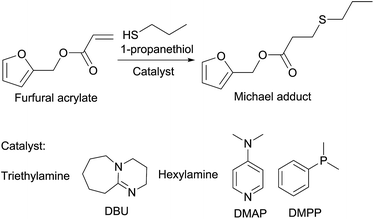 | ||
| Scheme 1 Addition of 1-propanethiol (1.1 eq.) to furfural acrylate (1 eq.) yielding the Michael adduct. | ||
| Entry | Catalyst | pKab | Reaction time (h) | Conversion (%) |
|---|---|---|---|---|
| a General conditions: furfural acrylate (0.6 mmol), 1-propanethiol (0.66 mmol) and tetrahydrofuran (1 mL) at the room temperature under an air atmosphere.b Refers to the pKa of the corresponding conjugates acid in H2O at 20 °C and pKa of 1-propanethiol was 10.2.c Hexylamine catalyst loading was 10 mol%.d Chloroform was used as the solvent.e DMPP catalyst loading was 0.5 mol%. | ||||
| 1 | TEA | 10.8 | 8 | 0 |
| 2 | DBU | 11.6 | 15 min | >99 |
| 3 | Hexylamine | 10.6 | 8 | >99 |
| 4 | Hexylamine | 10.6 | 4c | >99 |
| 5 | Hexylamine | 10.6 | 24d | 67 |
| 6 | DMAP | 9.7 | 5 | 44 |
| 7 | DMPPe | 6.49 | 15 min | >99 |
 | ||
| Scheme 2 (A) Nucleophile-catalysed mechanism for the thiol-Michael addition reaction, (B) potential side reaction using DMPP as the catalyst (R1 = C5H5O). | ||
On the other hand, a by-product was detected by GC analysis when hexylamine was used as the catalyst. As hexylamine concentration increased from 2 mol% to 10 mol%, the yield of the Michael adduct decreased from 98% to 92%. The molar mass of the by-product was 253 g mol−1, and was identified as the product of Aza-Michael addition between furfural acrylate and hexylamine (Fig. S3†). After the nucleophilic attack of the amine on the vinyl group generating a zwitterion, the proton transfer may have occurred to form the Aza-Michael adduct. Li et al. also observed that primary amines were able to react with the vinyl groups, generating stable species.9 Therefore, the results suggest that the use of hexylamine in a catalytic amount can minimize the side reaction in the thiol-Michael addition reaction, but may compromise the reaction rate. DMAP is another nitrogen-centered nucleophile that showed a relatively slow catalytic activity, as the conversion was 44% after 5 h reaction (entry 6). Both nucleophilicity and Lewis basicity of DMAP towards sp2 carbon centres are weaker than those of DBU,11 which explains the slow reaction rate. Phosphine catalysts are known to be very reactive for the thiol-Michael addition reaction.15 Hence, DMPP was investigated. Full conversion towards the expected Michael adduct was observed after 15 min (entry 7), and the yield of Michael adduct was over 99%. Although the phosphine catalyst has been reported to give rise to a side reaction in the thiol-Michael addition reaction,9 the faster reaction rate and the use of a catalytic amount of DMPP (0.5 mol%) are responsible for the quantitative yield in this study. Additionally, in order to exploit the potential side reaction between DMPP and the activated vinyl groups, DMPP (0.1 eq.) was mixed with furfural acrylate (1 eq.) in THF at room temperature for 6 h. Phosphine catalysed dimerization of furfural acrylate was the main product detected by GC analysis (Scheme 2B and Fig. S4†). The reaction proceeded slowly in comparison to the thiol-Michael addition reaction. Around 20% of furfural acrylate was converted after 6 h. Similarly, phosphine catalysed head-to-tail dimerization of methyl acrylate has been documented in other studies.16 In addition, furfural alcohol as a minor product was also observed by GC analysis. The possible pathway is shown in Scheme S1.†
Besides the observations mentioned above, the formation of disulfide was observed in the DBU and DMPP catalysed thiol-Michael addition reaction (Fig. S5†). Since the thiol-Michael addition reaction occurred under an air atmosphere, the formation of disulfide might be the result of a base catalysed oxidation of thiols.17 Therefore, it indicates that the reaction should be performed under an inert atmosphere. Otherwise, it can influence the structure of polymers synthesized by the thiol-Michael addition polymerization, particularly when the thiol groups are present in excess. In addition, DBU showed significantly higher reactivity toward the formation of disulfide than DMPP under same conditions. The presence of disulfide using DMPP is unexpected.18 DMPP can eliminate the disulfide via either preventing the aerial oxidation process or by acting as a reducing agent.7b,19 However, the reaction between the disulfide and phosphine catalyst has also been suggested to be a reversible disulfide metathesis process.20 In summary, these results suggest that DBU and DMPP are efficient catalysts to initiate the thiol-Michael addition reaction. Furthermore, using catalytic amount of DMPP allows the reaction to finish over a short period of time without the occurrence of side reactions. In this study, DMPP was selected to initiate the thiol-Michael addition polymerization of 2,5-furan diacrylate (2,5-FDA) and 1,6-hexanedithiol.
The synthesis of 2,5-FDA from HMF was performed via two steps (Scheme S2†). For the first step, an aldehyde group from HMF was efficiently reduced to the hydroxyl group using sodium borohydride (5 g, 96%) to yield BHF (Fig. S6†). Water has been successfully used as solvent for the reduction reaction under mild conditions,21 although organic solvents such as methanol, ethanol and THF have been reported in the literature.10,22 For the second step, the hydroxyl groups reacted with acryloyl chloride to generate acrylate groups (2 g, 56%). The structure of 2,5-FDA determined via 1H, 13C and HSQC NMR are presented (Fig. S7†).
The thiol-Michael addition polymerization of 2,5-FDA and 1,6-hexanethiol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was initiated via DMPP (0.5 mol%) in THF at room temperature under N2 atmosphere. The polymerization process was monitored by 1H NMR. As expected from the model reaction, the thiol-Michael addition polymerization proceeded quickly as the signals for acrylate groups disappeared within 15 min (from δ = 6.0 to 5.5 ppm, Fig. 1), and new signals appeared from δ = 3.0 to 2.0 ppm. The synthesized polymer (1 g, 95%) was soluble in common organic solvents, such as THF, chloroform and DMSO, but not soluble in hexane, water or methanol. The molecular weight determined by GPC showed that the number average molecular weight (
1) was initiated via DMPP (0.5 mol%) in THF at room temperature under N2 atmosphere. The polymerization process was monitored by 1H NMR. As expected from the model reaction, the thiol-Michael addition polymerization proceeded quickly as the signals for acrylate groups disappeared within 15 min (from δ = 6.0 to 5.5 ppm, Fig. 1), and new signals appeared from δ = 3.0 to 2.0 ppm. The synthesized polymer (1 g, 95%) was soluble in common organic solvents, such as THF, chloroform and DMSO, but not soluble in hexane, water or methanol. The molecular weight determined by GPC showed that the number average molecular weight (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n) of poly(β-thioether ester) was 19
n) of poly(β-thioether ester) was 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 529 g mol−1 after a 15 min polymerization reaction (polystyrene as a standard). According to the Carothers equation, the molecular weight of linear polymer can be controlled by introducing one monomer in excess.23 Moreover, the ends of the polymer chain are capped with the monomer in excess, which provides sites for further functionalization.8a Therefore, the polymerization process was performed with different monomer concentrations. As expected,
529 g mol−1 after a 15 min polymerization reaction (polystyrene as a standard). According to the Carothers equation, the molecular weight of linear polymer can be controlled by introducing one monomer in excess.23 Moreover, the ends of the polymer chain are capped with the monomer in excess, which provides sites for further functionalization.8a Therefore, the polymerization process was performed with different monomer concentrations. As expected, ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n of poly(β-thioether ester) decreased when the stoichiometric ratio of monomers was not equal to one (Table 2 and Fig. S8†). When the monomers containing acrylate groups were in excess, the molecular weight of polymers decreased more significantly, as compared to when thiol groups were in excess. The results of ATR-FTIR, 1H, 13C and HSQC NMR confirmed the expected structure of poly(β-thioether ester) (Fig. S9†).
n of poly(β-thioether ester) decreased when the stoichiometric ratio of monomers was not equal to one (Table 2 and Fig. S8†). When the monomers containing acrylate groups were in excess, the molecular weight of polymers decreased more significantly, as compared to when thiol groups were in excess. The results of ATR-FTIR, 1H, 13C and HSQC NMR confirmed the expected structure of poly(β-thioether ester) (Fig. S9†).
 | ||
| Fig. 1 1H NMR spectrum (blend of d-DMSO and CDCl3) of step-growth polymerization of 2,5-furan diacrylate and 1,6-hexanethiol in THF. (A) 0 min, (B) 15 min. | ||
Acrylate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SH SH |
Mn (g mol−1) | PDI |
|---|---|---|
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2609 | 1.7 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9438 | 1.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 529 529 |
2.0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 228 228 |
1.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
6077 | 1.8 |
The thermal transitions of poly(β-thioether ester) were studied by DSC (Fig. S10A†). The glass transition temperature (Tg) was −36.9 °C as determined in the second heating curve. The thermal stability of poly(β-thioether ester) was investigated using TGA under a nitrogen atmosphere (Fig. S10B†). The decomposition temperature of the polymer at 5% weight loss (T5%) was 236 °C. The maximum degradation rates were observed at 249.1 °C and 284.5 °C. The degradation observed at 249.1 °C was most probably due to the weaker C–S bonds present in the polymer chain.24
An organogel was synthesized in order to observe the Diels–Alder reaction between the furans and maleimides, as shown in Fig. S11 and Scheme S3.† Poly(β-thioether ester) was dissolved in chloroform followed by the addition of a bismaleimide cross-linker. The ratio of furan to maleimide was set at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The polymer solution was gently stirred and transitioned to a gel after 48 h. The material could return to its original form after heating at 140 °C for 60 min. The sol–gel process was successfully repeated three times.
1. The polymer solution was gently stirred and transitioned to a gel after 48 h. The material could return to its original form after heating at 140 °C for 60 min. The sol–gel process was successfully repeated three times.
Conclusions
In summary, it was demonstrated that three types of catalyst (base, primary amine and phosphine) were able to initiate the thiol-Michael addition reaction between furfural acrylate and 1-propanethiol. However, there were several side reactions for these catalysts, including the Aza-Michael addition reaction, the formation of disulfide, and the dimerization of furfural acrylate. The use of hexylamine in a catalytic amount could minimize the Aza-Michael addition reaction, but resulted in longer reaction times. The formation of disulfide was eliminated under an inert atmosphere. The dimerization of furfural acrylate was avoidable when using a catalytic amount of DMPP to initiate thiol-Michael addition reaction. As a result, DMPP efficiently catalysed the thiol-Michael addition polymerization to synthesize poly(β-thioether ester) from 5-HMF derivatives. The use of DMPP had several advantages including short reaction times, mild reaction temperature and high yield. Furthermore, the synthesis of a reversible organogel indicated that these polymers can be a starting point for engineering different materials properties using the Diels–Alder reaction.Acknowledgements
The financial support of the Natural Science and Engineering Research Council of Canada (NSERC) is gratefully acknowledged. We are also immensely thankful to Mr Yvan Gariépy for his support in our work. Furthermore, we really appreciate the help from Dr Chao-Jun Li, as well as Dr Robin Stein for NMR experiment setup.References
- A. Gandini, T. M. Lacerda, A. J. F. Carvalho and E. Trovatti, Chem. Rev., 2016, 116, 1637–1669 CrossRef CAS PubMed.
- (a) R.-J. van Putten, J. C. van der Waal, E. De Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed; (b) A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC; (c) A. S. Amarasekara, in Renewable Polymers, John Wiley & Sons, Inc., 2011, pp. 381–428, DOI:10.1002/9781118217689.ch9.
- (a) J. Chen, K. Li, L. Chen, R. Liu, X. Huang and D. Ye, Green Chem., 2014, 16, 2490–2499 RSC; (b) Y. J. Pagán-Torres, T. Wang, J. M. R. Gallo, B. H. Shanks and J. A. Dumesic, ACS Catal., 2012, 2, 930–934 CrossRef; (c) Y. Su, H. M. Brown, X. Huang, X.-d. Zhou, J. E. Amonette and Z. C. Zhang, Appl. Catal., A, 2009, 361, 117–122 CrossRef CAS; (d) J.-A. Chun, J.-W. Lee, Y.-B. Yi, S.-S. Hong and C.-H. Chung, Starch/Staerke, 2010, 62, 326–330 CrossRef CAS.
- (a) L. Hu, G. Zhao, W. Hao, X. Tang, Y. Sun, L. Lin and S. Liu, RSC Adv., 2012, 2, 11184–11206 RSC; (b) S. Dutta, S. De and B. Saha, ChemPlusChem, 2012, 77, 259–272 CrossRef CAS; (c) A. Gandini, Polym. Chem., 2010, 1, 245–251 RSC; (d) Y. Jiang, D. Maniar, A. J. J. Woortman, G. O. R. Alberda van Ekenstein and K. Loos, Biomacromolecules, 2015, 16, 3674–3685 CrossRef CAS PubMed.
- (a) J. Carlos Morales-Huerta, A. Martínez de Ilarduya and S. Muñoz-Guerra, Polymer, 2016, 87, 148–158 CrossRef CAS; (b) N. Yoshida, N. Kasuya, N. Haga and K. Fukuda, Polym. J., 2008, 40, 1164–1169 CrossRef CAS; (c) A. F. Sousa, C. Vilela, A. C. Fonseca, M. Matos, C. S. R. Freire, G.-J. M. Gruter, J. F. J. Coelho and A. J. D. Silvestre, Polym. Chem., 2015, 6, 5961–5983 RSC.
- B. D. Mather, K. Viswanathan, K. M. Miller and T. E. Long, Prog. Polym. Sci., 2006, 31, 487–531 CrossRef CAS.
- (a) D. P. Nair, M. Podgórski, S. Chatani, T. Gong, W. Xi, C. R. Fenoli and C. N. Bowman, Chem. Mater., 2014, 26, 724–744 CrossRef CAS; (b) J. W. Chan, B. Yu, C. E. Hoyle and A. B. Lowe, Polymer, 2009, 50, 3158–3168 CrossRef CAS; (c) J. Xu and C. Boyer, Macromolecules, 2015, 48, 520–529 CrossRef CAS; (d) A. H. Soeriyadi, G.-Z. Li, S. Slavin, M. W. Jones, C. M. Amos, C. R. Becer, M. R. Whittaker, D. M. Haddleton, C. Boyer and T. P. Davis, Polym. Chem., 2011, 2, 815–822 RSC.
- (a) J. Shin, H. Matsushima, J. W. Chan and C. E. Hoyle, Macromolecules, 2009, 42, 3294–3301 CrossRef CAS; (b) J. Vandenbergh, K. Ranieri and T. Junkers, Macromol. Chem. Phys., 2012, 213, 2611–2617 CrossRef CAS; (c) K. Dan and S. Ghosh, Polym. Chem., 2014, 5, 3901–3909 RSC; (d) K. Dan and S. Ghosh, Angew. Chem., Int. Ed., 2013, 52, 7300–7305 CrossRef CAS; (e) N. Zaquen, B. Wenn, K. Ranieri, J. Vandenbergh and T. Junkers, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 178–187 CrossRef CAS; (f) J. Vandenbergh, M. Peeters, T. Kretschmer, P. Wagner and T. Junkers, Polymer, 2014, 55, 3525–3532 CrossRef CAS; (g) J. Vandenbergh, G. Ramakers, L. van Lokeren, G. van Assche and T. Junkers, RSC Adv., 2015, 5, 81920–81932 RSC; (h) A. C. Pauly and F. di Lena, Polymer, 2015, 72, 378–381 CrossRef CAS; (i) S. Tomasi, R. Bizzarri, R. Solaro and E. Chiellini, J. Bioact. Compat. Polym., 2002, 17, 3–21 CrossRef CAS; (j) C. Xiao, J. Ding, L. Ma, C. Yang, X. Zhuang and X. Chen, Polym. Chem., 2015, 6, 738–747 RSC.
- G.-Z. Li, R. K. Randev, A. H. Soeriyadi, G. Rees, C. Boyer, Z. Tong, T. P. Davis, C. R. Becer and D. M. Haddleton, Polym. Chem., 2010, 1, 1196–1204 RSC.
- C. Zeng, H. Seino, J. Ren, K. Hatanaka and N. Yoshie, Macromolecules, 2013, 46, 1794–1802 CrossRef CAS.
- M. Baidya and H. Mayr, Chem. Commun., 2008, 1792–1794, 10.1039/B801811A.
- (a) J. W. Chan, C. E. Hoyle, A. B. Lowe and M. Bowman, Macromolecules, 2010, 43, 6381–6388 CrossRef CAS; (b) W. Xi, C. Wang, C. J. Kloxin and C. N. Bowman, ACS Macro Lett., 2012, 1, 811–814 CrossRef CAS.
- (a) C. Gimbert, M. Lumbierres, C. Marchi, M. Moreno-Mañas, R. M. Sebastián and A. Vallribera, Tetrahedron, 2005, 61, 8598–8605 CrossRef CAS; (b) C. Gimbert, A. Vallribera, J. A. Gladysz and M. Jurisch, Tetrahedron Lett., 2010, 51, 4662–4665 CrossRef CAS.
- J. W. Chan, H. Wei, H. Zhou and C. E. Hoyle, Eur. Polym. J., 2009, 45, 2717–2725 CrossRef CAS.
- B. S. Sumerlin and A. P. Vogt, Macromolecules, 2010, 43, 1–13 CrossRef CAS.
- (a) R. Tello-Aburto, A. N. Lucero and S. Rogelj, Tetrahedron Lett., 2014, 55, 6266–6268 CrossRef CAS; (b) Y. Feng and J. K. Coward, J. Med. Chem., 2006, 49, 770–788 CrossRef CAS PubMed.
- (a) A. L. Kieran, S. I. Pascu, T. Jarrosson, M. J. Gunter and J. K. M. Sanders, Chem. Commun., 2005, 1842–1844, 10.1039/B418811J; (b) S. Diemer, M. Kristensen, B. Rasmussen, S. Beeren and M. Pittelkow, Int. J. Mol. Sci., 2015, 16, 21858 CrossRef CAS PubMed; (c) E. Q. Rosenthal, J. E. Puskas and C. Wesdemiotis, Biomacromolecules, 2012, 13, 154–164 CrossRef CAS PubMed.
- T. Zhao, Y. Zheng, J. Poly and W. Wang, Nat. Commun., 2013, 4, 1873 CrossRef PubMed.
- J. W. Chan, B. Yu, C. E. Hoyle and A. B. Lowe, Chem. Commun., 2008, 4959–4961, 10.1039/B813438C.
- R. Caraballo, M. Rahm, P. Vongvilai, T. Brinck and O. Ramstrom, Chem. Commun., 2008, 6603–6605, 10.1039/B815710C.
- S. De, T. Kumar, A. Bohre, L. R. Singh and B. Saha, Bioorg. Med. Chem., 2015, 23, 791–796 CrossRef CAS PubMed.
- (a) B. Saha, C. M. Bohn and M. M. Abu-Omar, ChemSusChem, 2014, 7, 3095–3101 CrossRef CAS PubMed; (b) E. R. Sacia, M. Balakrishnan and A. T. Bell, J. Catal., 2014, 313, 70–79 CrossRef CAS.
- W. H. Carothers, G. L. Dorough and F. J. V. Natta, J. Am. Chem. Soc., 1932, 54, 761–772 CrossRef CAS.
- (a) J. Zhang, C. Pang and G. Wu, RSC Adv., 2016, 6, 11848–11854 RSC; (b) N. Lotti, V. Siracusa, L. Finelli, P. Marchese and A. Munari, Eur. Polym. J., 2006, 42, 3374–3382 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17532e |
| This journal is © The Royal Society of Chemistry 2016 |
