A floral variant of mesoporous carbon as an anode material for high performance sodium and lithium ion batteries
Abstract
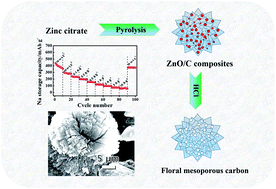
The floral variant of mesoporous carbon was simply prepared by direct pyrolysis of zinc citrate followed by washing with dilute hydrochloric acid. The unique floral microstructure endows the carbon with ultrahigh reversible capacity, excellent cycle stability and superior rate performance as an anode material for both sodium ion batteries and lithium ion batteries. The floral variant of mesoporous carbon exhibits a reversible sodium storage capacity as high as 438.5 mA h g−1 at a current density of 30 mA g−1 and retains a value of 68.7 mA h g−1 at an enhanced current density of 10 A g−1. Moreover, the floral mesoporous carbon can deliver a tremendous reversible capacity up to 1370 mA h g−1 at 50 mA g−1 as an anode for lithium ion batteries. It can output a high reversible capacity of 222 mA h g−1 even when being charged and discharged at 50 A g−1. Based on the astounding capacity and rate performance, the floral variant of mesoporous carbon can be regarded as one of the most promising anode materials for both sodium-ion and lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...