Corrosion behaviour of plasma electrolytic oxidation coated AZ91 Mg alloy: influence of laser surface melting pretreatment
Cancan Liuab,
Jun Liang*a,
Jiansong Zhou*a,
Qingbiao Liab,
Zhenjun Penga and
Lingqian Wanga
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, People's Republic China. E-mail: jliang@licp.cas.cn; jszhou@licp.cas.cn
bUniversity of Chinese Academy of Sciences, Beijing 100039, People's Republic China
First published on 18th July 2016
Abstract
Plasma electrolytic oxidation (PEO) was performed on a laser surface melting (LSM) modified AZ91 Mg alloy. The effect of LSM pre-treatment on the long-term corrosion resistance of the PEO coated AZ91 alloy was evaluated. Results showed that the LSM pretreatment had a negligible effect on the phase composition and microstructure of the PEO coatings. However, after LSM pretreatment, the long-term corrosion resistance of the PEO coated AZ91 alloy revealed a large enhancement. This was mainly ascribed to the improved corrosion resistance of the substrate resulting from the change of microstructure characteristics induced by the LSM treatment. This provided an alternative approach to improve the long-term corrosion resistance of the PEO coated Mg alloy by appropriate surface modified pretreatment of substrates.
1. Introduction
Due to low density and adequate specific strength, magnesium (Mg) alloys are attractive for the automotive, electronics and aerospace industries.1–3 However, a major hurdle restricting the extensive application of Mg alloys is their unsatisfactory corrosion properties.4,5 Many surface modification techniques have been developed as means for protection of Mg alloys, such as conversion coating, laser surface melting, plasma electrolytic oxidation, gas phase deposition and electrochemical plating.6–8 Of these techniques, plasma electrolytic oxidation (PEO) is one of the most common and efficient methods to improve the corrosion resistance of Mg alloys by producing a ceramic-like oxide coating on the surface. It has been proved that the corrosion resistance of Mg alloys can be remarkably enhanced by PEO treatment in the short term.9–12 Unfortunately, PEO coatings have inherent defects including micro-pores and micro-cracks. With long-term exposure, the corrosive medium could penetrate the coatings into the substrate through these defects, and thus made PEO fail locally.13–18A number of attempts have been made to enhance the long-term corrosion performance of PEO coatings on Mg alloys. One solution is to optimize preparation parameters of PEO coating including electrolyte and power supply to reduce the generation of defects and increase the content of stable compounds.19–21 However, this solution usually requires precise control of preparation parameters, which increases the difficulties in the production of PEO coatings. Another solution is to seal the micro-pores and micro-cracks of the PEO coatings by organic, inorganic or metallic top coatings through sealing, painting, electroless plating and other post treatments.22–25 Nevertheless, the inherent properties of the PEO coatings such as porosity, insulativity and scratch/wear resistance, may more or less be deteriorated or even shielded. Thus, it is important to develop alternative methods that can improve long-term corrosion performance of PEO coated Mg alloys without ruining the inherent properties of the coatings.
Many works have indicated that the corrosion properties of PEO coated Mg alloys had relation to microstructure characteristics of substrate.17,18 Tekin et al.17 compared the corrosion performance of PEO coating on E21, WE43 and AZ31 Mg alloys, and found that the corrosion resistance of PEO coated Mg alloys was closely related to the composition and microstructure of substrate alloys. Muhaffel et al.18 reported that the addition of alloying elements to the Mg substrate could enhance corrosion resistance of PEO coated Mg alloys. These investigations suggested that the corrosion properties of PEO coated Mg alloys can be modified by changing the substrate.
Generally, the microstructural characteristics of Mg alloys can be adjusted by heat treatment, addition of alloying elements and surface modification. The apparent advantage of surface modification method is that only the surface is modified while the bulk material remains unaltered. Laser surface melting (LSM) has a great potential to enhance surface properties of Mg alloys. It can not only homogenize and refine the microstructure, but also dissolve the second phases.26–28 These changes are conducive to improve the corrosion resistance of Mg alloys.29–34 Chen et al.35 indicated that duplex protection of LSM-PEO coating greatly improves corrosion resistance compared to the single PEO coatings in short term. The enhanced corrosion resistance was due to the higher thickness and the fewer defects of PEO coating after LSM pretreatment. On the other hand, Wang et al.16 reported that the PEO coating with LSM pre-treatment provide better long-term corrosion protection to AZ91 Mg alloy than the PEO coating alone. The authors deduced that the improvement of the corrosion resistance was attributed to both refined microstructure of substrate and reduced defects in PEO coating after LSM treatment, but they did not verify which one acts the crucial role. It is worthwhile to further explore the effect of LSM pretreatment on the corrosion protection of PEO coatings. In this work, AZ91 Mg alloy was treated by LSM process, followed by PEO. The main purpose of this work was to explore the reasons of the changed corrosion resistance of the PEO treated AZ91 alloy after the LSM treatment.
2. Experimental
2.1 Specimens preparation
AZ91 Mg alloy with chemical composition listed in Table 1, was supplied by Shanxi magnesium Co., Ltd (China). The specimens (50 mm × 50 mm × 10 mm) were extracted from the ingot and then sand blasted for the laser treatment. The LSM process was conducted using a DL-HL-10000 transverse-flow continuous-wave CO2 laser with a wave-length of 10.6 μm. Argon was used as a shielding gas to avoid oxidation during LSM process. The laser power and scan speed were set as 3500 W and 1000 mm min−1, respectively. The beam diameter of laser process was 3 mm. The overlapped track was 50% to form a uniformly melted layer. After that, the specimens were cut into rectangular plates of 30 mm × 20 mm × 10 mm. They were ground with SiC abrasive paper to 2000 grit and polished with Al2O3 paste to get a smooth surface for the following PEO treatment.| Alloy | Content of elements (wt%) | |||||
|---|---|---|---|---|---|---|
| Mg | Al | Zn | Mn | Si | Fe | |
| As-received | 90.42 ± 0.02 | 8.65 ± 0.15 | 0.64 ± 0.06 | 0.17 ± 0.09 | 0.04 ± 0.03 | 0.08 ± 0.02 |
| LSM | 88.80 ± 0.26 | 10.16 ± 0.09 | 0.75 ± 0.13 | 0.17 ± 0.07 | 0.10 ± 0.02 | 0.02 ± 0.02 |
The PEO process was conducted with a pulsed bi-polar power source. The coating was obtained in an alkaline electrolyte containing 10.0 g L−1 Na3PO4·12H2O (Alfa Aesar) and 1.0 g L−1 KOH. The pulse frequency, positive/negative voltage pulse ratio and duty cycle were 150 Hz, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 37.5%, respectively. A constant current density of 5.0 A dm−2 was used for 30 min. The temperature of the electrolyte solution was kept below 25 °C by a water cooling system. After the PEO treatment, the samples was washed thoroughly in distilled water and dried in warm air.
1.5 and 37.5%, respectively. A constant current density of 5.0 A dm−2 was used for 30 min. The temperature of the electrolyte solution was kept below 25 °C by a water cooling system. After the PEO treatment, the samples was washed thoroughly in distilled water and dried in warm air.
2.2 Microstructure characterization
For microstructure analysis, the AZ91 alloy were etched using nitric solution (10 mL 65.0 wt% nitric acid + 100 mL ethanol). The metallurgical microstructures of the LSM, PEO and LSM-PEO treated specimens as well as the as-received one were observed by a Zeiss Axio Imager.A2m microscope. The surface chemical composition and elemental distribution of the as-received and LSM treated specimens were analyzed using an energy-dispersive X-ray spectrometer (EDS, KEVER). The phase compositions of PEO coatings were investigated by a D/Max-2400 X-ray diffractometer (XRD, Cu Kα radiation) at a potential of 40 kV and current of 100 mA with a grazing angle of 5°. The surface and cross-section morphologies of coatings were observed by a scanning electron microscope (SEM, JSM-5600LV, JEOL, Japan). The thickness of coatings was measured by a minutest 1100 micro-processor coating thickness gauge (Elektro Physik Koln, Germany) with an accuracy of about ±1 μm.2.3 Electrochemical and immersion tests
Electrochemical corrosion tests were carried out using an Autolab PGSTAT302N electrochemical workstation to evaluate the corrosion behavior of the PEO and LSM-PEO treated specimens. A typical three-electrode cell system was used for each electrochemical test with the samples as the working electrode, a platinum plate as the counter electrode and an Ag/AgCl (saturated with KCl) electrode as the reference electrode. The specimens with 0.5 cm2 exposed area were immersed into 3.5 wt% NaCl solution at room temperature. Potentiodynamic polarization tests were performed at a scanning rate of 0.002 V s−1 after an initial 1 h exposure to achieve a stabilized open circuit potential. After certain immersion periods, EIS tests were operated at the open circuit potential with an AC amplitude of 10 mV. The frequency was ranged from 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 0.01 Hz. The degradation phenomena of the coatings can be analyzed by the change features in the impedance spectra. All the electrochemical tests were performed in triplicate to ensure the reproducibility of results.
000 to 0.01 Hz. The degradation phenomena of the coatings can be analyzed by the change features in the impedance spectra. All the electrochemical tests were performed in triplicate to ensure the reproducibility of results.
For immersion test, the specimens were sealed by polyurethane with the working surface exposed only, and then immersed in 3.5 wt% NaCl solution at room temperature. A digital camera was used to capture sample photos after certain immersion periods. After immersion test, the specimens were washed in clean running water and dried in warm air. The surface and cross-sectional corrosion morphologies of the specimens were observed by SEM.
3. Results
3.1 Microstructure and composition
The optical micrograph of the as-received AZ91 alloy is given in Fig. 1a. It can be seen that the as-received AZ91 alloy was composed of primary α-Mg and β-phase (Mg17Al12) surrounded by eutectic α-Mg as well as Al8Mn5 phase. Similar observation has been reported by others.27,32 Obviously, the amount and size of β-phase was much larger than that of Al8Mn5 phase in AZ91 alloy. Fig. 1b and c display cross-sectional and surface microstructures of LSM treated alloy. The LSM treatment produced a homogeneous melted layer with the average depth of 1.3 mm. The intermetallic compounds (β-phase and Al8Mn5 phase) were remarkably refined by the LSM treatment. Besides, the refined β-phase uniformly distributed in the α-Mg matrix to form a nearly continuous network-like structure.Fig. 2 reveals the surface element distribution of the as-received and LSM treated alloy. The Al and Mn elements in the modified layer distributed more uniformly than that in the as-received one, which confirmed the dissolution and redistribution of intermetallic compounds. Besides, the Al content in the melted layer was larger than that in the substrate, as shown in Table 1. This was probably due to the preferential evaporation of Mg from the melt pool during LSM process.26,36 Overall, the LSM treatment led to the enrichment of Al element, the refinement and redistribution of intermetallic compounds in the modified layer.
 | ||
| Fig. 2 SEM micrographs of the as-received (a) and LSM treated (b) AZ91 alloy and corresponding element distribution. | ||
3.2 Coating analysis
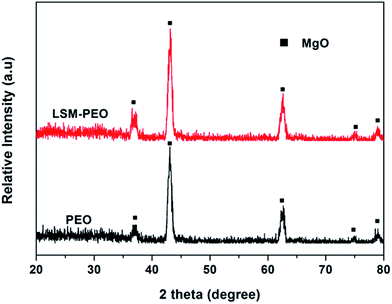
Fig. 3 displays XRD patterns of the PEO coatings on the as-received and LSM treated alloy. It can be found that MgO was the main constituent for two coatings, regardless of the LSM pretreatment. The diffraction peaks of Mg3(PO4)2 were not observed, which was probably because it exist in the form of noncrystal phosphate under the preparation parameters used in this work.37Fig. 4 shows the surface morphologies of PEO coatings on the as-received and LSM treated AZ91 alloy. The two coatings were similar in number and size of micro-pores. There also existed some micro-cracks on the surface of the coatings. The cross-section micrographs in Fig. 5 indicated the similar construction of coatings, consisting of the outer porous outer layer and the compact inner layer. Moreover, the average thickness of PEO coating on the as-received alloy was equal to that on the LSM treated alloy (about 21 μm). However, when the cross-sectional morphologies of the specimens were further investigated by optical microscope (Fig. 6), it can be found that the substrates underneath two coatings exhibited different microstructures. In summary, the LSM pretreatment had a little effect on the coating but changed the coating/substrate interface characteristics under the preparation condition in this work.
 | ||
| Fig. 4 Surface SEM micrographs of PEO coatings on the as-received (a) and LSM (b) treated AZ91 alloy. | ||
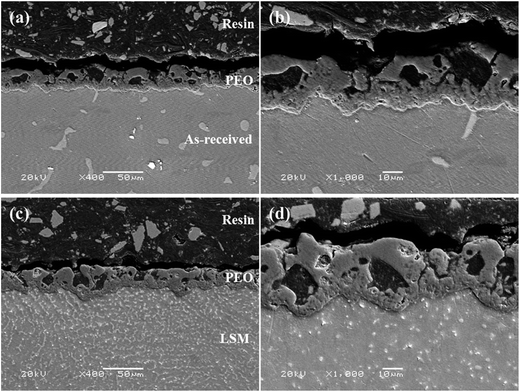 | ||
| Fig. 5 Cross-sectional SEM micrographs of the PEO (a and b) and LSM-PEO (c and d) treated AZ91 alloy. | ||
3.3 Effect of LSM pretreatment on the corrosion behavior of PEO treated alloy
The corrosion resistance of the PEO and LSM-PEO treated specimens was evaluated by the potentiodynamic polarization test in a 3.5 wt% NaCl solution. The potentiodynamic polarization curves of specimens are given in Fig. 7. The polarization curve of the as-received AZ91 alloy is also inserted for comparison. The corrosion potential (Ecorr) and the corrosion current density (icorr) derived from the polarization curves are listed in Table 2. It can be seen that the icorr values of PEO treated specimens decreased by one order of magnitude as compared with that of the as-received one, indicating that the PEO treatment greatly enhanced the corrosion resistance of AZ91 alloy. In addition, the LSM-PEO treated specimen displayed a nearly four times lower icorr than the PEO treated one. Obviously, the LSM pretreatment was helpful to increase the corrosion resistance of the PEO treated AZ91 alloy. | ||
| Fig. 7 Potentiodynamic polarization curves of the PEO and LSM-PEO treated AZ91 alloy in 3.5 wt% NaCl solution. | ||
| Specimen | Ecorr (mV vs. Ag/AgCl) | icorr (A cm−2) |
|---|---|---|
| As-received | −1349 | 2.84 × 10−6 |
| PEO | −1383 | 2.90 × 10−7 |
| LSM-PEO | −1423 | 7.39 × 10−8 |
The EIS tests were applied to analyze the degradation process of the PEO and LSM-PEO treated alloy. Fig. 8 displays the EIS plots of specimens during immersion in 3.5 wt% NaCl solution for different time. The impedance data at low frequency (|Z|f→0 Hz) in the Bode plot is generally considered as an indication of the corrosion resistance of the specimen.38 In this work, the corrosion resistance of the specimen was represented by the value of |Z|f=0.01 Hz in Bode impedance plots. The values of |Z|f=0.01 Hz as a function of immersion time are presented in Fig. 9. For the PEO coated alloy, the |Z|f=0.01 Hz values decreased remarkably with the immersion time from 0.5 h to 5 h (Fig. 9), indicating that the specimen underwent a quick deterioration of the corrosion resistance. Up to 10 h immersion, the inductive loop appeared in the Bode plots (Fig. 8b). On the other hand, the |Z|f=0.01 Hz values of the LSM-PEO coated AZ91 alloy maintained relatively high levels (Fig. 9) during the 40 h immersion process. The |Z|f=0.01 Hz value was found to increase slightly after immersion for 20 h, which was attributed to that the hydrated products Mg(OH)2 from MgO could partially block the defects and act as protective barrier to corrosive solution.15 Up to 50 h immersion, the |Z|f=0.01 Hz value was still high, but inductive loop could be found in the phase angle Bode plot (Fig. 8d). This phenomenon suggested that the localized corrosion occurred for the PEO coated AZ91 alloy after 50 h of immersion. However, the corrosion damage was too slight to affect the resistance value in Blot plot. This phenomenon corresponded to corrosion feature of the LSM-PEO coated AZ91 alloy. Subsequently, the |Z|f=0.01 Hz value dropped in the order of magnitude. It was clear that the long-term corrosion resistance of the PEO coated alloy was remarkably improved by the LSM pretreatment.
 | ||
| Fig. 8 Blot plots of PEO (a and b) and LSM-PEO (c and d) treated AZ91 alloy after immersion in 3.5 wt% NaCl solution for different time. | ||
 | ||
| Fig. 9 The impedance modulus at 0.01 Hz (|Z|f=0.01 Hz) of the PEO and LSM-PEO coated AZ91 alloy as a function of immersion time. | ||
The long-term corrosion behavior of specimens was further investigated by immersion tests. Fig. 10 presents the surface appearances of the PEO and LSM-PEO treated specimens during immersion tests. For the PEO treated alloy, slight corrosion pits appeared on the sample surface after immersion for 82 h (Fig. 10a). Subsequently, corrosion dramatically aggravated as evidenced by the broadening damage area. Finally, most part of the sample surface was corroded. Conversely, as presented in Fig. 10b, there was no apparent corrosion damage on the LSM-PEO coated alloy during the whole immersion process. Only several tiny white bumps could be observed after 587 h immersion. The results of immersion tests further proved the positive effect of LSM pretreatment on the long-term corrosion resistance of the PEO treated alloy.
 | ||
| Fig. 10 Photographs of the PEO (a) and LSM-PEO treated (b) AZ91 alloy after immersion in 3.5 wt% NaCl solution for different time. | ||
To more clearly observe the corrosion features, the surface and cross-sectional micrographs of the PEO and LSM-PEO treated specimens after 587 h immersion were analyzed. The surface of PEO treated alloy was characterized by discrete corrosion damage (Fig. 11a). All discrete corrosion areas were adjacent to β-phases and developed along the boundaries of the β-phases (Fig. 11b). Moreover, the corrosion depth along the cross-section was much larger than the thickness of the PEO coating. This manifested that the coating on the top of the corrosion area was completely destroyed. In the case of LSM-PEO coated alloy, there was no obvious corrosion damage on the surface and only a small raised area was observed (Fig. 11c). The cross-section micrographs in Fig. 11d showed that the raised area was related to the corrosion products formed at the interface between the coating and substrate. The corrosion products lifted the PEO coating but did not completely damage the coating, resulting in the occurrence of the small raised areas on the surface. However, many micro-cracks was also formed in the coating, which corresponded to the decrease of the corrosion protection performance of the coating evaluated by EIS measurements. Based on the discussion above, the corrosion products forming in the interface could exert a stress which lifted or damaged the PEO coating.
 | ||
| Fig. 11 The surface and cross-section micrographs of the PEO (a and b) and LSM-PEO treated (c and d) AZ91 alloy after immersion in 3.5 wt% NaCl solution for 587 h. | ||
4. Discussion
Based on the above results, it can be concluded that LSM treatment greatly improved the long-term corrosion resistance of the PEO coated AZ91 alloy. It is generally believed that the improved long-term corrosion resistance of the PEO coated AZ91 alloy should be strongly related to the corrosion protection property of the PEO coatings.16 The corrosion protection property of the PEO coating is determined by the coating characteristics, such as microstructure, composition and thickness. However, aforementioned results showed that the LSM pretreatment exerted a negligible influence to the microstructure, composition and thickness of the PEO coatings. Thus, the PEO coatings would provide more or less the same corrosion protection to the substrates, regardless of the LSM treatment. The PEO treated alloy had to be considered as a system of coating and substrate. The reason of enhanced long-term corrosion resistance of LSM-PEO treated alloy can only be the change of substrate induced by LSM treatment. In the following context, the effect of the LSM treatment on the corrosion behavior of alloy substrate was investigated to make clear how the LSM treatment influence the corrosion behavior of alloy substrate and further influence the corrosion behavior of PEO treated AZ91 alloy.4.1 Effect of LSM treatment on the corrosion behavior of substrates
Fig. 12 exhibits the photographs taken of as-received and LSM treated AZ91 alloy during immersion tests. As given in Fig. 12a, the surface of the as-received alloy featured local corrosion. Visible corrosion pits appeared on the surface of the as-received alloy after immersion for 5.5 h. The number and area of corrosion pits increased as time proceeded. Up to 30 h, the AZ91 alloy suffered extensive corrosion damage. After the LSM treatment, the time of starting apparent corrosion damage was retarded to 22 h of immersion (Fig. 12b). In addition, bits of small corrosion pits were found after immersion for 30 h. This proved the positive effect of LSM treatment on the corrosion resistance of AZ91 alloy. | ||
| Fig. 12 Photographs of the as-received (a) and LSM treated (b) AZ91 alloy after immersion in 3.5 wt% NaCl solution for different time. | ||
Fig. 13 exhibits the surface and cross-sectional morphologies of the as-received and LSM treated alloy after 30 h immersion. For the as-received alloy, discrete corrosion damage appeared on the surface (Fig. 13a). It can be seen that the corrosion pattern of the PEO treated alloy was similar to that of the as-received one, suggesting the corrosion pattern of the PEO treated alloy was influenced by the substrate. Besides, corrosion deeply extended into the substrate and the corrosion depth was in the range of 50–120 μm (Fig. 13b). Interestingly, the corrosion progressed as dissolution of the α-Mg matrix (dark grey) while impeded in the region of β-phase (light grey), resulting in the corrosion developing heterogeneously in the substrate. On the contrary, the corrosion depth of the LSM treated alloy was much smaller than that of the as-received alloy (Fig. 13c and d). Meanwhile, the corrosion was restricted to the region with refined β-phases (Fig. 13d).
 | ||
| Fig. 13 Surface and cross-section micrographs of the as-received (a and b) and LSM treated (c and d) AZ91 alloy after immersion in 3.5 wt% NaCl solution for 30 h. | ||
The different corrosion behaviors between the as-received and LSM treated specimens can be elucidated below according to their microstructure characteristics. The as-received AZ91 alloy was composed of α-Mg, eutectic mixture (α + β) and Al8Mn5 phases. In the as-received alloy, isolated coarse β-phase preferentially served as an effective galvanic cathode to accelerate corrosion process of the α-Mg matrix as exposed to corrosive solution.39 After LSM treatment, the enriched Al element and refined intermetallic compound in the modified layer lowered the corrosion susceptibility of α-Mg matrix. Moreover, the distance of refined β-phases was small and the β-phases formed a nearly continuous network-like structure. In this condition, the β-phases mainly served as a barrier to impede corrosion process of α-Mg matrix. Thus, after LSM treatment, the time of starting the corrosion of AZ91 alloy was retarded and the content of localized corrosion was reduced.
4.2 Corrosion mechanism
It was known that MgO, the main constituent of the PEO coatings in this work, could degrade gradually in the neutral NaCl solution by hydration to form thermodynamically more stable Mg(OH)2.40 However, the hydration of MgO didn't destruct the structural integrity of the coating itself and kept to provide corrosion protection in a longer immersion time if there was no other external destruction. This can be proved by the result of immersion tests of the LSM-PEO coated AZ91 alloy specimen (Fig. 10b and 11d). In contrast, the PEO treated alloy suffered severe corrosion damage (Fig. 10a). Therefore, it can be deduced that the corrosion of the PEO treated alloy and the destruction of the PEO coatings was crucially related to the corrosion of underneath substrate. Accordingly, the corrosion mechanisms of the PEO and LSM-PEO treated specimens can be proposed as follow.When the PEO treated specimens were immersed in corrosive solution, the corrosive solution would penetrate into the coating/substrate interface through defects and contact with the substrate. For the PEO coated alloy, the corrosion of substrate would quickly occur due to low corrosion resistance. With the immersion time, corrosion of substrate rapidly aggravated because of the accelerating effect of β-phases, and thus more and more corrosion products formed at the coating/substrate interface. The volume increase of the corrosion products would exerted a stress to PEO coating, which lifted the coating, and in some case, led to peel off of the coating (Fig. 11b).14,18 Thus, the corrosion solution was much easier to reach the substrate, and more corrosion products formed due to the corrosion of the substrate. And so on, severe corrosion damage appeared on the PEO treated alloy in short term.
In case of the LSM-PEO coated AZ91 alloy, as the corrosion solution arrived at the interface, the initiation of corrosion of the substrate was retarded effectively due to the enhanced corrosion resistance after the LSM treatment. On the other hand, the corrosion of the substrate was lightened and only restricted to superficial area. As a result, only small amounts of corrosion products were formed and accumulated at the coating/substrate interface, which led to the coating lifted a little but didn't peel off. In this way, the LSM pretreatment helped to enhance the long-term corrosion resistance of the PEO treated alloy.
5. Conclusions
1. Under the preparation condition in this work, MgO was found to be the main component of PEO coatings on AZ91 alloy, as-received or LSM treated. Moreover, the LSM treatment had little effect on the microstructure and thickness of PEO coating. Nevertheless, the LSM pretreatment remarkably improved the long-term corrosion resistance of the PEO treated AZ91 alloy.2. Failure of the PEO coating was mainly caused by volume expansion due to the accumulative corrosion products in the interface. The LSM treatment could produce a uniform modified layer on AZ91 alloy. After LSM treatment, the time of starting the corrosion of AZ91 alloy was retarded and the content of corrosion damage was mitigated, due to the combination of reduced the corrosion susceptibility of α-Mg matrix and the barrier effect of β-phases. Thus, in the same immersion time, a much smaller amount of the corrosion products accumulated at the interface of the LSM-PEO treated alloy, compared to the PEO treated alloy. In this way, the LSM pretreatment helped to enhance the long-term corrosion resistance of the PEO treated AZ91 alloy.
Acknowledgements
The financial support from National Natural Science Foundation of China (Grant No. 51241006) and the “Hundred Talents Program” of Chinese Academy of Sciences (J. Liang) was gratefully acknowledged.References
- B. L. Mordike and T. Ebert, Mater. Sci. Eng., A, 2001, 302, 37 CrossRef.
- Y. Kojima, Mater. Trans., 2001, 42, 1154 CrossRef CAS.
- M. K. Kulekci, Int. J. Adv. Manuf. Tech., 2008, 39, 851 CrossRef.
- G. L. Song and A. Atrens, Adv. Eng. Mater., 1999, 1, 11 CrossRef CAS.
- G. L. Song and A. Atrens, Adv. Eng. Mater., 2003, 5, 837 CrossRef CAS.
- A. Atrens, G. L. Song, M. Liu, Z. M. Shi, F. Y. Cao and M. Dargusch, Adv. Eng. Mater., 2015, 17, 400 CrossRef CAS.
- B. Li and Y. Han, RSC Adv., 2015, 5, 46109 RSC.
- H. Zhao, S. Cai, Z. T. Ding, M. Zhang, Y. Li and G. H. Xu, RSC Adv., 2015, 5, 24586 RSC.
- Y. Mori, A. Koshi, J. Liao, H. Asoh and S. Ono, Corros. Sci., 2014, 88, 254 CrossRef CAS.
- J. Liang, L. Hu and J. Hao, Appl. Surf. Sci., 2007, 253, 4490 CrossRef CAS.
- L. Pezzato, K. Brunelli, E. Napolitani, M. Magrini and M. Dabalà, Appl. Surf. Sci., 2015, 357, 1031 CrossRef CAS.
- J. Zhuang, Y. Guo, N. Xiang, Y. Xiong, Q. Hu and R. Song, Appl. Surf. Sci., 2015, 357, 1463 CrossRef CAS.
- R. Arrabal, A. Pardo, M. C. Merino, M. Mohedano, P. Casajus, E. Matykina, P. Skeldon and G. E. Thompson, Corros. Sci., 2010, 52, 3738 CrossRef CAS.
- J. Liang, P. Bala Srinivasan, C. Blawert, M. Stormer and W. Dietzel, Electrochim. Acta, 2009, 54, 3842 CrossRef CAS.
- J. Liang, P. Bala Srinivasan, C. Blawert and W. Dietzel, Electrochim. Acta, 2010, 55, 6802 CrossRef CAS.
- L. Wang, J. Zhou, J. Liang and J. Chen, J. Electrochem. Soc., 2014, 161, C20 CrossRef CAS.
- K. C. Tekin, U. Malayoğlu and S. Shrestha, Surf. Coat. Technol., 2013, 236, 540 CrossRef CAS.
- F. Muhaffel, F. Mert, H. Cimenoglu, D. Höche, M. L. Zheludkevich and C. Blawer, Surf. Coat. Technol., 2015, 269, 200 CrossRef CAS.
- H. P. Duan, C. W. Yan and F. H. Wang, Electrochim. Acta, 2007, 52, 3785 CrossRef CAS.
- L. Pezzato, K. Brunelli, S. Gross, M. Magrini and M. Dabala, J. Appl. Electrochem., 2014, 44, 867 CrossRef CAS.
- H. S. Ryu, S. J. Mun, T. S. Lim, H. C. Kim, K. S. Shin and S. H. Hong, J. Electrochem. Soc., 2011, 158, C266 CrossRef CAS.
- U. Malayoglu, K. C. Tekin and S. Shrestha, Surf. Coat. Technol., 2010, 205, 1793 CrossRef CAS.
- T. S. Lim, H. S. Ryu and S. H. Hong, J. Electrochem. Soc., 2013, 160, C77 CAS.
- X. Fan, Y. Wang, B. Zou, L. Gu, W. Huang and X. Cao, Appl. Surf. Sci., 2013, 277, 272 CrossRef CAS.
- G. Y. Liu, S. W. Tang, J. Hu, Y. F. Zhang, Y. M. Wang and F. Liu, J. Electrochem. Soc., 2015, 162, C426 CrossRef CAS.
- D. Dubé, M. Fiset, A. Couture and I. Nakatsugawa, Mater. Sci. Eng., A, 2001, 299, 38 CrossRef.
- Y. C. Guan, W. Zhou, H. Y. Zheng and Z. L. Li, Appl. Phys. A: Mater. Sci. Process., 2010, 101, 339 CrossRef CAS.
- P. C. Banerjee, R. K. Singh Raman, Y. Durandet and G. McAdam, Corros. Sci., 2011, 53, 1505 CrossRef CAS.
- G. Abbas, Z. Liu and P. Skeldon, Appl. Surf. Sci., 2005, 247, 347 CrossRef CAS.
- J. D. Majumdar, R. Galun, B. L. Mordike and I. Manna, Mater. Sci. Eng., A, 2003, 361, 119 CrossRef.
- R. K. Singh Raman, S. Murray and M. Brandt, Surf. Eng., 2007, 23, 107 CrossRef.
- A. E. Coy, F. Viejo, F. J. Garcia-Garcia, Z. Liu, P. Skeldon and G. E. Thompson, Corros. Sci., 2010, 52, 387 CrossRef CAS.
- C. Taltavull, B. Torres, A. J. Lopez, P. Rodrigo, E. Otero, A. Atrens and J. Rams, Mater. Des., 2014, 57, 40 CrossRef CAS.
- C. C. Liu, J. Liang, J. S. Zhou, L. Q. Wang and Q. B. Li, Appl. Surf. Sci., 2015, 343, 133 CrossRef CAS.
- L. X. Chen, Y. Liu, Z. Y. Liu, X. Y. Zhao and W. Li, Mater. Corros., 2015, 66, 963 CrossRef CAS.
- Y. C. Guan, W. Zhou, Z. L. Li and H. Y. Zheng, Appl. Surf. Sci., 2009, 255, 8235 CrossRef CAS.
- J. Liang, B. G. Guo, J. Tian, H. W. Liu, J. F. Zhou, W. M. Liu and T. Xu, Surf. Coat. Technol., 2005, 199, 121 CrossRef CAS.
- S. J. Xia, R. Yue, R. G. Rateick Jr and V. I. Birss, J. Electrochem. Soc., 2004, 151, B179 CrossRef CAS.
- G. L. Song, A. Atrens and M. Dargusch, Corros. Sci., 1999, 40, 249 Search PubMed.
- C. C. Liu, J. Liang, J. S. Zhou, Q. B. Li and L. Q. Wang, Appl. Surf. Sci., 2016, 382, 47 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |