Graphite pencil electrodes as electrochemical sensors for environmental analysis: a review of features, developments, and applications
Abdel-Nasser Kawde
*,
Nadeem Baig
and
Muhammad Sajid
Department of Chemistry, King Fahd University of Petroleum and Minerals, Dhahran 31261, Saudi Arabia. E-mail: akawde@kfupm.edu.sa
First published on 19th September 2016
Abstract
Graphite pencil electrodes (GPEs) are carbon-based electrodes that are recognized by their low cost, simplicity, commercial availability, ease of modification and disposability. GPEs are attractive substrates for electrochemical sensing because of their unique feature of “disposability” compared to other commonly used carbon-based electrodes. Mechanically rigid GPEs are easy to modify and miniaturize. The sensitivity and selectivity of GPE toward certain analytes can be enhanced by applying different modification materials. The primary focus of this review article is to highlight the applications of GPEs in the analysis of inorganic and organic pollutants in different environmental matrices. This review gives a brief overview of the various types of inorganic and organic pollutants and their impact on the environment. The key features of modified GPEs that enhance their electrocatalytic activity toward detection of certain target analytes are critically appraised. In the end, we summarize the current status, weaknesses and future prospects of GPE based sensors for environmental analysis.
1. Introduction
The Scopus database reveals that it was probably 1960 when for the first time a graphite electrode obtained from an HB pencil was used as the working anode in polarography without any modification.1 Though this publication cites another work published in 1954, where a graphite pencil electrode was used as a reference electrode, this cited work is difficult to trace. Until the middle of the 1990s, there were only a few reports on modified GPEs for sensing of some analytes2–4 e.g. an antibody immobilized GPE was utilized as a direct potentiometric immunoelectrode for detection of atrazine.3 However, these reports have yet not described any disposable and renewable GPEs. During the same period, some disposable electrodes such as metal electrodes or carbon materials deposited metal substrates were being used but their disposability was only good from an economic perspective and the issue of occupational and environmental safety was another aspect to be considered because of their applications in trace level detection of toxic substances.To the best of our knowledge, renewable GPE was for the first time reported in 1997 for stripping voltammetry of cadmium and lead.5 Although for writing purposes, the renewable graphite pencils were introduced many years ago, but their potential as low-cost, readily available, and the renewable electrode was not realized up till 1997. Some notable works that were conducted in following years include detection of RNA and DNA,6 labels free detection of DNA hybridization,7,8 and analysis of trace metals.9
The feature of renewable writing pencils where lead can be extruded to any desired length from the holder is very well utilized in GPEs. After each electrochemical measurement, the used surface of GPE can be easily renewed by removing the extruded part. Moreover, the sensing area of the pencil exposed in the solution can be easily controlled according to the requirement of analysis. From the green chemistry perspective, the only small material is used as an electrode and can be readily disposed of after use.
GPE is a subtype of the graphitic electrodes that has specialized characteristics of the high surface area, good conductivity and ease to use. However, the uniqueness of GPE is credited to some of its specific properties like it is cheap,10 commercially available and easily disposable. Moreover, GPEs are mechanically rigid, easy to modify and miniaturize.11 GPEs are most attractive electrodes because they offer a simple and faster surface renewal compared to other commonly used electrodes which involve tedious surface polishing procedures. Because of renewable surfaces, GPEs are expected to give reasonably reproducible results. Additionally, GPEs show strong adsorption properties, low background current, and wide potential window.12
As a result of industrial revolution and urbanization, human environment is badly affected by the variety of inorganic and organic pollutants. These pollutants have been identified as a serious challenge for the survival of human and wildlife. Hence, some of these pollutants have been regulated by national and international environment protection agencies and their allowable limits have been defined. No doubt, last few decades have witnessed great progress in the instrumentation for analysis of a variety of inorganic and organic pollutants but the high cost of these sophisticated instruments itself a big challenge in low resource setups. In addition to cost, these instruments are not suitable for in field applications. Simple, low-cost, efficient, portable and commercially available instruments or electrochemical sensors are desired to fulfill the demand of worldwide monitoring of pollutants in different matrices.13 Because of their simplicity, low-cost, large-scale availability, GPE based electrochemical sensors can be a good choice for environmental analysis in limited resource setups.
In the recent years, there has been observed an increasing trend for the applications of GPE based electrodes in the environmental analysis. To the best of our knowledge, this is the first review article that covers environmental analytical applications of GPE. Hence, this review gives brief description of
(i) Highly toxic pollutants from inorganic and organic origin.
(ii) Applications of GPE based electrochemical sensors for detection of these pollutants in environmental matrices.
(ii) Properties of the GPE and modified GPEs which enhance the sensitivity and selectivity of the electrode toward particular target species.
Fig. 1 gives a pictorial description of the features of GPE that have been covered in this review article.
2. Electrochemical analysis of inorganic pollutants at GPE
The presence of metals, metalloids and other inorganic pollutants in environmental matrices is the most important environmental problem of the modern age. Both natural and anthropogenic sources contribute significant amounts of inorganic pollutants in the environment. In the coming sections, we enlist and highlight the importance of studying inorganic pollutants in the environment. The applications and potential of GPE based sensors for detection of inorganic pollutants are critically discussed in each section.2.1. Metallic pollutants
Heavy metal pollution is a global environmental challenge. Heavy metals are distributed in the environment as a result of their increased applications in domestic, industrial and agricultural processes. The heavy metals such as mercury, lead, cadmium, chromium and arsenic are considered priority pollutants and have been regulated by a number of national and international environmental agencies. Metallic pollutants are recognized as “systematic pollutants” and they cause various ailments in the human body including damage to different organs. Some metals have been classified as carcinogens (well known or suspected) by USEPA.14 Metal toxicity to an individual depends on upon many factors such as kind of exposure (environmental or occupational), dose and period of exposure and nature of the metal itself. However, it is obvious that continuous exposure to low-level concentrations of these pollutants may lead to serious health implications. From the polluted environment, these metals may enter to food chain and thus to the bodies of humans, other animals, and aquatic organisms. Therefore, sensitive analytical methods are needed to measure the levels of heavy metals in the environment. In order to deal with a global environmental challenge posed by heavy metal pollution, simple, efficient, portable and cost-effective analytical methods are always desired. The last few decades have witnessed some major advancements in analytical instrumentation for analysis of heavy metals. The instruments like atomic absorption spectroscopy (AAS) and inductively coupled plasma-mass spectrometry (ICP-MS) have been widely used for determination of metals in different matrices. Although, these instruments are highly sensitive and selective, but some major issues are associated with them. They are very expensive, require a number of consumables, and their portability for in field applications is a big challenge. Before the analysis, extended procedures of sample preparation are needed. Moreover, analysis is destructive, time-consuming and expert operators are required. Electrochemical methods for detection of heavy metals are simple, efficient, cost-effective, non-destructive and do not require extended sample preparation procedures for common environmental matrices such as water and soil. Electrochemical methods can provide adequate sensitivity and selectivity for detection of metals. In addition, these methods can be used for speciation and multi-elemental analysis. GPE based electrodes are very cheap and easily available and can be used to perform cost-effective analysis in limited resources setups. Hence, in the following paragraphs, we described some applications of GPE and modified-GPE for sensing of metals in environmental samples. Table 1 lists applications of GPE based electrodes for detection of inorganic analytes in different environmental matrices.| Electrode | Analyte | Matrix | Medium/pH | Technique | Linear range | LOD | Detection in real samples | Ref. |
|---|---|---|---|---|---|---|---|---|
| PSS/CnP/GPE | Cu(II) | Mineral, sea and river water | 0.1 M KCl/3.0 | DPASV | — | 0.11 μg L−1 | Yes | 21 |
| Cu-P4R/PPy/GPE | Cu(II) | Tap water | pH range 4.0–6.0 | Potentiometry | 1 × 10−5 M to 5 × 10−2 M | 6.7 × 10−6 M | Yes | 22 |
| Cu–Car/PA/GPE | Cu(II) | Green tea leaves, black currants, sour cherry juice | 6.0 | Potentiometry | 5 × 10−6 to 1 × 10−1 M | 2 × 10−6 M | Yes | 23 |
| P4VP/GPE | Cd(II) | Soil, tea, cabbage, liver, kidney, heart | 6.4 | Potentiometry | 1 × 10−7 to 1 × 10−1 M | 2.51 × 10−8 M | Yes | 15 |
| Bi-NPs/puMWCNT/GPE | Pb(II) | — | 0.1 M NaOAc/4.5 | DPV | 0.4–10.8 μmol L−1 | 1.7 nmol L−1 | No | 17 |
| DPC/PANI/GPE | Cr(VI) | Tap water | 2.0 | Potentiometry | 1 × 10−6 to 1 × 10−1 M | 8 × 10−7 M | Yes | 18 |
| Tar/PPy/GPE | Zn(II) | Barley flakes, rice, dry milk | 5.0 | Potentiometry | 1.0 × 10−5 to 1.0 × 10−1 M | 8.0 × 10−6 M | Yes | 19 |
| NG/PG/BiE | Pb(II), Cd(II), Zn(II) | Tap water | 0.1 M acetate buffer/4.6 | SWASV | 2–20 μg L−1, 10–100 μg L−1 | 0.17 & 0.22 μg L−1, 0.09 & 0.09 μg L−1, 0.13 & 0.20 μg L−1 | Yes | 20 |
| Ag/FeOOH/GPE | H2O2 | Disinfectant | 0.1 M PBS/7.2 | Amperometry | 0.03–15.00 mM | 22.8 μM | Yes | 32 |
| PtNPs/GPE | H2O2 | — | 0.1 M PB/7.0 | Amperometry | 10–110 μM | 3.6 μM | No | 30 |
| PdNPs/GPE | H2O2 | — | 0.1 M PB/7.0 | Amperometry | 10–140 μM | 0.045 μM | No | 31 |
| PVF+/MWCNTs/GPE | NO2− | Mineral water | 0.05 M PB/7.4 | DPV | 1–400 μM | 0.1 μM | Yes | 33 |
| Poly(PyY)/GPE | NO2− | Salami | 0.1 M PBS/4.0 | Amperometry | 1–100 μM | 0.5 μM | Yes | 34 |
| Au NPs/GPE | N2H4 | Drinking water | 0.1 M PB/5.0 | SWV/amperometry | 0.05–1000 μmol L−1, 25–1000 μmol L−1 | 42 nmol L−1, 3.07 μmol L−1 | Yes | 12 |
An ISE was fabricated by electropolymerization of 4-vinyl pyridine on 2B pencil graphite as ionosphere for Cd2+. The electrode gave good limit of detection (LOD) down to 2.5 × 10−8 M and relatively a broad pH range 4.0–7.5 for cadmium measurement. The major advantages of this modification are cheap material, easy modification and potential to apply in in situ measurements of cadmium in real samples. The developed electrode was used to determine Cd2+ by using the potentiometric method in different environmental (soil), food (tea, vegetables) and biological (cow liver, kidney, heart and chicken liver and heart) samples.15
There are only few reports which describe simultaneous detection of more than one metal ions on modified GPEs. The Nafion graphene nanocomposite pencil graphite bismuth film electrode (NG-PG-BiE) was used for simultaneous detection of Pb2+, Cd2+ and Zn2+ ions in tap water using square wave anodic stripping voltammetry. The NG-PG-BiE exhibited detection limits of 0.17, 0.09 and 0.13 μg L−1 for Zn2+, Cd2+ and Pb2+ much lower than below the USEPA allowable limits of 5 mg L−1, 5 μg L−1 and 15 μg L−1 for Zn2+, Cd2+ and Pb2+ respectively.20
Ion-selective electrodes have shown some exciting applications in the current years because of their ability to analyze the target ions rapidly in small sample specimen with high accuracy and reproducibility. Potentiometric based ion selective electrodes are convenient because of their low cost, non-destructive analysis, and not affected by turbid or colored samples. Polypyrrole conducting polymer doped with Ponceau 4R azo dye based modified GPE was used for selective potentiometric determination of Cu2+ in water. The pyrrole monomer was electrochemically polymerized on GPE in the presence of Ponceau 4R azo dye as a dopant. The presence of the dopant in electropolymerization generates selective recognition sites in the formed polymer, and these sites can interact selectively with Cu2+ ions. This modified electrode showed good LOD values down to 6.7 μM. LODs remained unchanged for the shelf life of 21 days, and a slight change was observed after 60 days. The one limitation for this electrode is that it is only suitable in acidic medium (pH, 5).22 Another similar work is reported where GPE modified with conducting polyaniline doped with copper carmoisine dye complex was used for potentiometric monitoring of Cu2+ in green tea leaves, black currants, and sour cherry juice. This modified electrode showed a very fast response (20 s) and good limit of detection 2.0 μM.23
2.2. Comparison of GPE based sensors and other instrumental methods for analysis of metals
Here it will be suitable to compare the GPE based electrochemical methods and other instrumental methods which are used for detection of metallic impurities in environmental samples. Flame atomic absorption spectrometry (FAAS) is one of the major technique used for determination of metals such as cadmium, lead, zinc and copper in different matrices. The disadvantages that are associated with FAAS are mainly related to matrix interference on the analyte signal and higher limit of detections than the normal concentration of metals present in real samples. Thus, removal of interferences and improvement in LOD is achieved through separation and preconcentration of the metals prior to analysis.24 This kind of sample preparation itself is a tedious job because multistep processes are involved that may comprise reaction (derivatization), extraction, separation, preconcentration and determination. Moreover, the accuracy and reliability of sample preparation assisted FAAS depends upon the number of steps involved. It is matter of fact that these sample pretreatment methods have made trace element assays more convenient, but more often they require large sample or reagent volumes (several tens to hundreds of milliliters) to get high enrichment factors or preconcentration factors. However, when it is difficult to obtain such large sample volumes, these techniques led to low enrichment factors and high detection limits.25 When dealing with trace elements, total content analysis as well as speciation analysis both are very important. Trace metals exist in different oxidation states and some metal species have been reported to show varying toxicities in their different oxidation states, for example, hexavalent chromium is much more toxic than trivalent chromium.26 In order to find out the role of trace element species in different diseases, recent research in area of trace element analysis is shifting from total content to speciation analysis. Hence, very efficient extraction methods that would be capable of selective speciation and then highly selective detectors are desired in analytical instruments.27In general, no specialized sample preparation is necessary for electrochemical methods. In addition to that electrochemical methods are cost and time effective. For the trace elemental analysis, the speciation can be easily performed by simply altering the detection potential. Moreover, very low LODs can be achieved by electrochemical methods without performing any sample preconcentration. When particularly talking about graphite pencil electrodes, they are easily available in the market and cost much lesser than other carbon or metal-based electrodes. However, the automation of GPE based electrochemical methods presents a challenge that need to be addressed for successful commercialization of such analytical tools. Table 2 compares GPE based sensors with other instrumental methods.
| Metal | Matrix | Sample preparation | Detection method | Detection technique | LOD | Ref. |
|---|---|---|---|---|---|---|
| Cadmium | Water | Pyrolysis vapor generation | Spectroscopic | AFS | 2.2 ng mL−1 | 77 |
| Water and food | Modified MNPs based SPE | Spectroscopic | FAAS | 3.71 ng mL−1 | 78 | |
| Water and food | Nanomagnetic task specific ionic liquid as a selective sorbent in SPE | Spectroscopic | FAAS | 0.5 ng mL−1 | 24 | |
| Water and food | Pyridine-functionalized magnetic nanoporous silica material | Spectroscopic | FAAS | 0.04 ng mL−1 | 79 | |
| Soil, tea, cabbage, liver, kidney, heart | — | P4VP/GPE based electrochemical | Potentiometry | 2.51 × 10−8 M | 15 | |
| Lead | Water and human air | Magnetic ion-imprinted polymer (MIIP) nanoparticles | Spectroscopic | GFAAS | 2.4 ng L−1 | 80 |
| Water samples | Air-assisted liquid–liquid extraction | Spectroscopic | FAAS | 1.36 ng mL−1 | 81 | |
| — | — | Bi-NPs/puMWCNT/GPE based electrochemical | DPV | 1.7 nmol L−1 | 17 | |
| Copper | Water, food and hair samples | Switchable solvent-based liquid phase microextraction | Spectroscopic | FAAS | 1.8 ng mL−1 | 82 |
| Water samples | Temperature-assisted dispersive liquid–liquid microextraction | Spectroscopic | GFAAS | 1.82 ng L−1 | 83 | |
| Mineral, sea and river water | — | PSS/CnP/GPE based electrochemical | DPASV | 0.11 μg L−1 | 21 | |
| Tap water | — | Cu-P4R/PPy/GPE based electrochemical | Potentiometry | 6.7 × 10−6 M | 22 | |
| Green tea leaves, black currants, sour cherry juice | — | Cu–Car/PA/GPE based electrochemical | Potentiometry | 2 × 10−6 M | 23 | |
| Zinc | Water samples | Temperature-assisted dispersive liquid–liquid microextraction | Spectroscopic | GFAAS | 0.89 ng L−1 | 83 |
| Water samples | Solid phase extraction | Spectroscopic | FAAS | 0.24 ng mL−1 | 84 | |
| Water samples | Surfactant-based dispersive liquid–liquid microextraction | Spectroscopic | FAAS | 85 | ||
| Barley flakes, rice, dry milk | — | Tar/PPy/GPE based electrochemical | Potentiometry | 8.0 × 10−6 M | 19 |
2.3. Other inorganic pollutants
In addition to the metals, other substances such as hydrazine, hydrogen peroxide, sulfides, cyanide, perchlorate, oxides of nitrogen and sulfur have a great environmental impact as inorganic pollutants. Few of these have been electrochemically detected in different environmental matrices using GP based electrodes. A brief account of these pollutants is provided in coming sections.Later on, Chelladurai Karuppiaha et al. have reported highly sensitive sensor Au NPs/AG/SPCE for detection of hydrazine. This sensor was more sensitive compared to simple Au NPs/GPE. The sensitivity of the SPCE was enhanced by coating the dispersed graphite and dried cast graphite was later on electrochemically activated. On AG/SPCE the Au NPs were electrochemically deposited and this activation process of graphite and the presence of Au NPs on the surface of SPCE made the sensor highly sensitive towards hydrazine and very low LOD down to 0.57 nM achieved.28 However, in case of Au NPs/GPE no activation of graphite was involved prior to modification with Au NPs.
Some sensitive analytical methods based on a modification of GPE have been developed for detection of H2O2. NPs and nanomaterials modified GPE showed good electrocatalytic activity toward H2O2 detection. Platinum NPs modified GPE was fabricated for highly sensitive detection of H2O2. This modification was achieved by a very simple process which involves dipping of GPE in an aqueous solution of 0.5 mM (NH4)2PtCl4 and 0.55 mM ascorbic acid followed by heating at 75 °C for 15 min. The electrode gave excellent tolerance against interferes and LOD of 3.6 μM was achieved.30 In another work, palladium NPs modified GPE was used for sensing of H2O2. This method also involves the facile synthesis of Pd NPs on the surface of GPE. In this Pd NPs were prepared by the very simple approach by adding aqueous solution of ascorbic acid (AA) to aqueous solution of (NH4)2PdCl4 and stirring it for 15 min at room temperature. The color change of (NH4)2PdCl4 from pale yellow to dark brown was indicative of NPs formation. GPE was then incubated in a solution of NPs for 15 min at room temperature. The H2O2 was determined in the aqueous medium using the amperometric technique. The modified electrode was capable of detecting H2O2 at nanomolar levels by amperometric method, and LOD of 45 nM was obtained which was much lower than the LOD obtained at bare GPE under the same set of experimental conditions (0.58 mM).31
In another work, Ag/FeOOH nanocomposites were immobilized on GPE for amperometric detection of H2O2. The rationale behind employing FeOOH nanomaterials was their high surface area which can provide better support for AgNPs immobilization. The developed electrode showed good long-term stability and was able to retain 90% of the signal after 3 weeks of modifications. LOD of 22.8 μM was achieved and method was used to measure H2O2 in disinfectants.32
3. Electrochemical analysis of organic pollutants at GPE
The rapid industrialization in the modern era resulted in the release of the toxic organic pollutants into the environment. Although most of the industries try to detoxify or degrade these organic pollutants by physical, chemical or biological means but still significant amounts of these pollutants are released into the environment. Organic pollutants are famous by different names including “emerging organic pollutants”, “priority pollutants”, “endocrine disrupting compounds” etc.35 A continuous monitoring of these hazardous chemicals is necessary to take precautionary measures for sustainability of the healthy environment. In the coming section, we discuss the applications of GPE based sensors for detection of different classes of organic pollutants in environmental matrices.3.1. Phenolic compounds
Phenolic compounds are very common and abundant environmental pollutants. Most of the phenolic compounds induce very severe health problems, and they have been classified as hazardous wastes and virulent pollutants by the United State Environmental Protection Agency.36 They adversely affect human health by accelerating weight loss and weariness. Phenols are reported to cause respiratory cancer, cardiac and immune system disorders in case of severe exposures. The applications of GP based electrodes for high-sensitivity detections of different phenols are given in following sections.3.2. Organic dyes
A lot of dyes are released into the water as a residue during the coloring process in the textile industry and severely pollutes the environment. In dying process, almost 10 to 15% and in some conditions, up to 50% dyes are wasted and released to the aquatic bodies. Azo dyes are the commonly used dyes and contain azo group (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). The presence of azo dyes in the water has adverse effect on the aquatic life and the human health.46
N–). The presence of azo dyes in the water has adverse effect on the aquatic life and the human health.46
 | ||
| Fig. 2 Schematic representation of electrochemical detection of SN using molecularly imprinted PPy films on GPE. Reproduced from 48 with permission. Copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
3.3. Pesticides
The pesticides represent a broad class of chemicals which is further divided into insecticides, herbicides, fungicides, nematicides, molluscicides, and rodenticides. No doubt, pesticides have played a major role in increasing food production worldwide but at the same time, their excessive use has led to serious health threats to the human and wildlife. The excessive use of pesticides results in their accumulation in food, soil, and water. It is reported that almost one million people die or suffer per year from chronic diseases resulted from exposure to pesticides.50 Chlorpyrifos is the commonly used organophosphate pesticide, and its exposure can initiate disorders in neurological and the autoimmune system. A method has been developed for the trace level determination of the chlorpyrifos by fabrication the molecular imprinted polymer modified pencil graphite electrode. The impedance technique has been used for the measurement of the chlorpyrifos in the soil, tape water and corn leaves. The impedance increases as the concentration of the chlorpyrifos increases in the sample and wide linear range was observed from 20 to 300 μg L−1 with a detection limit of 4.5 μg L−1.513.4. Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are widespread environmental pollutants. PAHs have been added into the environment by human activities and also by natural incidents like volcanic eruption, forest fires, burning of fossils and the petroleum fuel. These compounds are considered highly toxic due to their mutagenic and carcinogenic behavior. The PAHs carcinogenicity has been related to the number of benzene rings in their structures and their transformation into the reactive electrophilic intermediates metabolites. The mammalian converts these compounds into the detoxification products in the liver and these metabolized products when excreted are more genotoxic and reactive. The PAH metabolites are responsible for carcinogenic process by covalently binding to the cellular DNA. These aromatic compounds become activated in the presence of sunlight and cellular destruction may start if skin comes into contact with these compounds in the presence of light.| Electrode | Analyte | Matrix | Medium/pH | Technique | Linear range | LOD | Detection in real samples | Ref. |
|---|---|---|---|---|---|---|---|---|
| pCu/GPE | 4-Nitrophenol | — | 0.1 M acetate buffer/4.8 | Amperometry | 50–850 μM | 1.91 μM | No | 40 |
| ETGPE | Bisphenol A | Tap and river water | 0.1 M PBS/2.0 | ASDPV | 0.05–5.0, 5–10 μM | 0.0031 μM | Yes | 45 |
| DNA/MWCNTs-PDDA/GPE | Chrysoidine | Food products | 0.5 M acetate buffer/4.8 | DPV | 0.05–15.00 μg mL−1 | 0.03 μg mL−1 | Yes | 47 |
| MIP/PPy/GPE | Sulfanilamide | Blood serum, ground water | 0.04 M BR buffer/2.0 | DPV | 5.0 × 10−8 to 1.1 × 10−6 M, 1.1 × 10−6 to 48 × 10−6 M | 2.0 × 10−8 M | Yes | 48 |
| PGPE | 4-Nonylphenol, 4-octylphenol, 4-tert-octylphenol | Industrial water | PBS/7.4 | DPV | 1.2–94.0 μM, 0.6–78 μM, 2.38–243 μM | 0.42 μM, 0.25 μM, 0.77 μM | Yes | 11 |
| MIP/PPy/GPE | Chlorpyrifos | Tap water, soil, corn leaves | 0.1 M KCl | Impedance | 20–300 μg L−1 | 4.5 μg L−1 | Yes | 51 |
| PANI/MWCNTs/GPE | Bisphenol A | Extracted water from baby bottles | 0.1 M glycine–NaOH/10.6 | Amperometry | 1–400 μM | 10 nM | Yes | 44 |
| Ds-DNA/GPE | Sudan II | Chili sauce, ketchup sauce | 0.5 M acetate buffer/4.8 | DPV | 0.5–6.0 μg mL−1 | 0.4 μg mL−1 | Yes | 49 |
| PGPE | Sudan II | Chili sauce, ketchup sauce | 0.1 M PBS/4.8 | ASDPV | 0.0015–0.30 μg mL−1 | 0.00007 μg mL−1 | Yes | 49 |
| GPE | Thiourea | Waste water | PB/12.0 | SWV | 6.3–30 μM | 1.29 μM | Yes | 86 |
| Ds-DNA/GPE | 7,12-Dimethylbenz[a]anthracene | Urine | 0.1 M acetate buffer/4.8 | SWSV | 2–10 nM | 0.194 nM | Yes | 52 |
| PGPE | Benzo[a]pyrene | Urine | 0.1 M acetate buffer/4.8 | ASWV | 0.25–1.25 μM | 0.027 μM | Yes | 53 |
4. A brief overview of materials used for modification of GPE
In order to enhance their electrocatalytic activity toward certain inorganic or organic analytes, GP based electrodes were modified with different materials. These modification materials include metal nanoparticles, conducting polymers and carbon-based materials. In the following sections, we have discussed modification materials and their potential to increase electrocatalytic activity.4.1. Nanoparticles
Nanoparticles (NPs) are characterized by their better physical, chemical, and electronic features than their bulk counterparts. It is the nature of the material used for synthesis that determines properties of NPs. Commonly, NPs are synthesized by chemical reduction of metal salts in the presence of a stabilizer. Stabilizers are attached to the surface of NPs and play a major role in defining their charge, solubility, and stability. NPs are extremely sensitive to the changes occurring on their surface. NPs modified electrodes may result in enhanced electron kinetics due to following(i) High surface area.
(ii) Enhanced mass transport rates.
(iii) Better control of NP surface.
(iv) Functionalization of NPs with selective groups.
Recently, metal NPs (MNPs) have drawn significant attention in electrocatalysis because of their unique chemical and electronic conduct. The analytes that show sluggish electron kinetics at bare electrodes were reported to have reasonably enhanced electrocatalytic activity at MNP's modified electrodes. In addition, MNPs modified electrodes showed good peak separations for compounds having very close oxidation potentials.
Recently, the very simple procedure was adopted for immobilization of Au NPs on GPE for detection of hydrazine. The beauty of this modification is in the process that involves direct attachment of Au NPs on the surface of GPE without the use of any cross-linkers. Fig. 3 shows SEM images of Au NPs modified GPE at different magnification levels, and Fig. 4 clearly shows enhanced response at Au NPs modified GPE compared to bare GPE for detection of hydrazine.12 Similarly, in another report, platinum modified GPE was synthesized by one-step reaction that involves reduction of metal salt while dipping the GPE in reaction media.30 There are some examples where MNPs are used in combination with other materials such as CNTs. Such combinations are thought to contribute to sensing properties of electrodes through synergistic effect.
 | ||
| Fig. 3 FE-SEM images at two different magnifications, 2 μm and 200 nm of gold nanoparticle-modified GPE. Modified and reprinted from 12 with permission. Copyright 2013 Elsevier. | ||
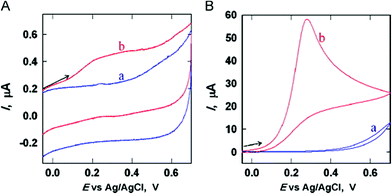 | ||
| Fig. 4 CVs in 0.1 mol L−1 PBS (pH 7) in the absence (A) or presence (B) of 0.5 mmol L−1 hydrazine at a bare GPE (a) and at a AuNP-GPE (b). Scan rate: 100 mV s−1. Modified and reprinted from12 with permission. Copyright 2013 Elsevier. | ||
4.2. Conducting polymers
Different types of polymers are used in electrochemical sensing applications. Overall, these polymers can be categorized into four classes i.e. electroactive, polyelectrolyte, coordinating and biological polymers. The selection of a certain polymer for electrode modification is mainly dictated by the nature of the target analytes. The electrodes can be modified with polymers using different strategies. The frequently used methods include spin coating, dip coating, and electropolymerization. Conducting polymers allow the incorporation of counter ions or other functional moieties into the structure of polymers. In this way, modified electrodes can be made selective against target analytes. Their electronic properties have similarity with metals. The polymer coated electrodes can help in avoiding interferences because selective coating materials will distinguish between the analyte and other species via hydrophilic and hydrophobic interactions, electrostatic affinities and ion exchange capabilities.The use of Ponceau 4R azo dye doped polypyrrole modified GPE for selective potentiometric determination of Cu2+ in water is an example of the polymer based ion selective electrode. In this work, the pyrrole monomers were electrochemically polymerized on GPE in the presence of Ponceau 4R azo dye as a dopant. The application of dopant in electropolymerization generates selective recognition sites in the coating which interact selectively with Cu2+ ions.22 Similarly, there are numerous other examples where polymer modified GPEs were employed as ISE for analysis of different metals.15,19,23 MIPs48 and polymer nanocomposites33,44 are also used for modification of GPE for different sensing applications.
4.3. Carbon nanotubes
Carbon nanotubes (CNTs) have gained a great attraction since its discovery in 1991 by Iijima. CNTs being a sp2 allotrope of carbon could be divided into two types (a) single-wall carbon nanotubes (SWCNTs) and (b) multiwall carbon nanotubes (MWCNTs). SWCNTs could be considered as the rolling of the single layer graphene while MWCNTs is the rolling of the multilayer graphene.54 SWCNTs and MWCNTs could be synthesized by vapors deposition methods, electrical arc discharge, and laser vaporization.55 The rapid development has been witnessed in the fabrication of the CNTs based sensor from past few years. Many studies have been reported in which CNTs based sensors has shown great electrocatalytic properties towards analytes. CNTs are considered as good electrode material due to fast charge transfer and also shown the compatibility and synergistic effect with the other electrode materials.54H. Safardoust-Hojaghan et al. has introduced a new device consist of purified MWCNTs and Bi NPs nanocomposite which was immobilized on the surface of GPE. The response of the Bi NPs modified GPE was increased by adding the purified MWCNTs. The MWCNTs increased the surface area with Bi NPs and facilitated the fast charge transfer. The electrochemical impedance study has shown the MWCNTs with Bi NPs have reduced the charge transfer resistance significantly.17 In another work, the MWCNTs was used in combination with electroactive polymer poly(vinyl ferrocenium). PVF+/MWCNTs/GPE was fabricated by single step electrooxidation of PVF/MWCNTs at +0.7 V for determination of nitrite. MWNCNTs and poly(vinyl ferrocenium) have increased the sensitivity of the GPE electrode for nitrite determination compared to bare GPE and PVF+/GPE.33 MWCNTs were also used for the GPE based biosensors. A. Ensafi et al. reported work in which dsDNA was immobilized on the surface of MWCNTs-PDDA/GPE to develop a biosensor for the sensitive sensing of banned dye, chrysoidine. PDDA is a positive charge electrolyte which adsorbs or wraps the MWCNTs when sonicated together. This process leads to positive charge nanocomposite which provided a good effective surface area for the loading of dsDNA.47 In another work, the MWCNTs with polyaniline nanorods was used for the surface modification of the GPE. PANI rods alone on the GPE surface has given broad and low oxidation peak current compared to MWCNTs which describes MWCNTs has more fast charge transfer capability. The combination of both PANI and MWCNTs has shown excellent sensitivity towards bisphenol A.44
4.4. Pretreatment
This is a very common method to enhance the sensitivity of the carbon-based electrodes. This method is widely being used for increasing the sensitivity of the graphite pencil electrode. During electrochemical pretreatment, the electrode surface was cleaned, and some oxygen-containing groups also formed on the surface of GPE. The enhancement of the current could be attributed to the formation of oxygen-containing functional groups like carboxylic acid, phenolic, and carbonyl. In this process might be some graphite oxide films are also formed on the GPR surface which is also responsible for the current enhancement for the target analytes.56 Electrochemical pretreatment is attractive choice due to the following reasons.57• Very simple.
• Less time-consuming.
• No complex material required.
• More applicable compared other modification materials.
For pretreatment, the number of electrolytes is being used like NaOH, the pretreated electrode was used for the determination of Sudan.49 E. Keskin et al., pretreated the pencil electrode by dipping the electrode in DMSO/0.1 M LiClO4 mixture at +1.6 V for sensing of benzo[a]pyrene.53 Recently, the surface of the graphite pencil electrode was charged by pretreating the GPE in NaOH solution. The charged surface was used for the self-electropolymerization of the phenol on the electrode surface for the sensitive sensing of phenol. It is a very simple method for the determination of phenol.37 The effect of different pretreatment solutions on the response of GPE surface charging for detection of phenol is shown in Fig. 5.
 | ||
| Fig. 5 (A) SWVs obtained from a 2 μM phenol solution in 0.1 M phosphate buffer, pH 7.2, in the presence of the uncharged (a) and charged GPEs in various 0.1 M media: (b) NaCl, (c) HCl, (d) Na2HPO4, and (e) NaOH solutions. (B) The corresponding histogram. Reproduced from37 with permission Copyright 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
5. Comparison of GPE with other electrodes
Graphite pencil electrode is still an emerging electrode. There are few characteristics which make it unique over other carbon and metallic based electrodes and a good candidate for electrochemical sensing.This single use electrode has the advantage to make the surface foul free.37,58 Any electrode could be a single use electrode, but the cost is an issue. Glassy carbon electrode (GCE), Au and Pt disc electrode could not be employed as disposable electrodes due to high cost and their manufacturing style. Due to this reason, GCE, Au, and Pt disc electrodes are being used for multiple times after polishing the surface by number of different ways to get rid of adsorbed materials and to improve the electrochemical behavior.59–61 GPE could be a single used electrode as the new surface is readily available after the measurement. However, screen printed electrode (SPE) is well established single use electrode, but it is costly compared to GPE. SPE surface cleaning is usually not required, but polishing could have some positive effect.62 For carbon paste electrode (CPE) fabrication, a typical preparation is needed which is time-consuming.63
Control of sensing area is another attractive characteristic which is associated with GPE58 and it is not much feasible for GCE, CPE and the SPEs where the active surface is provided by the supplier. In case of GPE the length of the extruded graphite pencil could be adjusted according to the requirement. Recently, the GPE was used for the sensing of α-naphthol. The response of the bare GPE towards α naphthol was compared with GCE, CPE, Au and Pt disc electrode and it was more sensitive.64 Casting modification of GPE is difficult and challenging due to its elongated shape and small diameter compared to GCE, CPE, SPE, Au and Pt disc electrode.
The comparison of LODs for the detection of same analyte at different bare and modified electrode is nearly impossible task. The similar modifications of various electrodes can give real insight of the sensitivity of the electrodes toward certain analytes. However, it is rare to find similar modification on all electrodes for detection of same analyte. Moreover, the LOD of the H2O2 sensing on the different modified electrodes is compared (Table 4). While making this comparison, it was tried to choose the modification as similar as possible. Pd NPs/GPE has shown good response towards H2O2 (ref. 31, 60 and 65–67) compared to closely modified electrodes. All these characteristics made GPE a valuable electrode for electrochemical sensing.
| Sr# | Characteristic | GPE | GCE | CPE | SPE | Au disc E | Pt disc E |
|---|---|---|---|---|---|---|---|
| 1 | Availability | Easily available | Easily available | Need preparation | Easily available | Easily available | Easily available |
| 2 | Cost | Very cheap | Costly | Average cost | Costly (due to single use) | Costly | Costly |
| 3 | Disposable (single use) | Yes | No | No | Yes | No | No |
| 4 | Surface polishing | Not required58 | Required59 | Required (removal and smoothing of surface)63 | Not required | Required60 | Required61 |
| 5 | Control of sensing area | Yes | No | No | No | No | No |
| 6 | Sensitivity (α-naphthol) | Very good64 | Good64 | Good64 | — | Low64 | Low64 |
| 7 | Casting modification | Difficult | Easy59 | Easy87 | Easy88 | Easy89 | Easy90 |
| 8 | (Modifications)/LOD analyte (H2O2) | (Pd NPs-GPE)/0.045 μM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
(Pd NPs-GCE)/0.34 μM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 65 |
(HRP-Au NPs/CCPE)/6.3 μM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66 |
(Pt–PdBNC/SPGFE)/0.87 μM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
((MWCNT/Ag nanohybrids) modified gold electrode)/0.5 μM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
— |
5.1. Attractive features and challenges of graphite pencil electrodes
GPE possesses many attractive and unique features which make it an excellent choice for electrochemical sensing. These features are summarized below.• A unique feature is the provision of the renewable surface after each measurement68 just like the dropping mercury electrode. This feature makes the electrode surface foul free. But in the case of most of other electrodes like GCE, after each electrochemical measurement, a number of by-products are formed which can adhere to the surface of the electrode and could compromise the sensitivity of the electrode. However, here in case of GPE, the part of the used electrode can be easily removed, and the new surface is readily available.
• The surface polishing is generally not required prior to use.69
• The GPE is easily available70 as it is widely used for writing purposes as a graphite pencil.
• It is also disposable70 like screen-printed electrode. However, it is extremely low cost71 compared to disposable SPE.
• Handling of GPE is relatively easy, and due to its elongated shape, the direct electro-modification methods are more facile.72
• GPE is found more sensitive compared to GCE64,71 (greater sensitivity is due to the presence of porosity on the surface of GPE).
• The control of the sensing area is also possible by changing the extruded pencil length.
Despite its excellent behavior and the advantages, the GPE is facing few problems. Still, it is not a well-established electrode, and it is not prepared for electrode applications, due to this reason, sometimes the electrode composition may vary. This could affect the sensor behavior. Such issues can be fixed by preparing the graphite pencil typically for electrode purposes. The modification by casting method is also challenging due to the smaller diameter of a pencil.
Although there are some challenges associated with GPE, the GPE can be an excellent commercial electrode due to the above attractive features for the study of environmental pollutants. It has the capability to overcome the regeneration problem of the other solid state electrodes.69
6. Future scope and outlook
Point of care (POC) testing has become the most well-known way of diagnosis in clinical analysis, food safety, and the environment. The main advantage of POC lies in the fact that it provides results in very short time compared to centralized laboratories. These devices are helpful in making quick decisions.The water that is considered to be a matrix of life has been highly polluted with inorganic and organic impurities in different parts of the globe due to the industrial revolution. This fact has already been indicated by a number of published reports.73. Similarly, thousands of publications talk about analytical method development for analysis of inorganic and organic pollutants in water, food74 and biological samples.75 However, POC like devices that can readily analyze water or other environmental matrices for the presence of inorganics or organics is still not available to the public. Hence, there is very high stress on existing analytical technologies to meet the demands of low cost, availability, sensitivity and applicability in on field analysis.
GPE based electrochemical devices can have the potential to serve in POC applications. Their low cost, easy availability, and trait of disposability make them quite fit for POC based devices. Despite low cost and commercial availability of GP based electrodes, the commercialization of readily available GPE sensors for real life samples is still at some distance from the reality. Every lab prepared prototype cannot be brought to the market without considering its suitability for the desired assay, study of consumables and acceptability by the consumers. Although electrochemical methods involving modified GPEs demonstrate a grade of maturity good enough to implement them in real analytical applications but up till now, their applications are limited to proof of concepts. For a proof of concept, different materials such as nanoparticles, conductive polymers, and carbon-based materials have been used to modify the surface of GPEs. However, which of them can be employed for mass production of modified GPEs for certain analysis will be dictated by cost, ease of synthesis and stability of the material over the electrode surface. Moreover, the integration of such modified electrodes into miniaturized systems is nothing less than a challenge as commercialization needs careful designing of the product that should be compatible with market demands. Thus, these challenges need to be addressed in future.
7. Conclusive remarks
In the area of environmental analysis, a growing variety of low-cost analytical sensors is always welcomed. Useful sensors are recognized not only by their cost but the simplicity of fabrication and operation. The sensors which can serve in point of care or in field applications even for qualitative or semi-quantitative analysis are paramount for elevating the quality of life through quick analysis.76 GPE based electrodes have been widely used for quantitative determination of inorganic and organic, environmental pollutants. GPEs are inexpensive and commercially available electrodes that can compete with other expensive metallic and non-metallic electrodes. GPE offers renewable surface, which can be easily modified with a number of materials including nanoparticles and conducting polymers, CNTs, and pretreatment. Modification of GPE with nanomaterials or direct formation of NPs on GPE surface enhances its surface area, which in turn, results in high sensitivity and improved electrochemical activity toward detection of target species. GPE based electrodes are easy to miniaturize. Due to high selectivity and sensitivity, portability, rapid analysis, and ease of availability, GP based electrodes offer excellent opportunities for decentralized environmental analytical labs. These electrodes can also provide an opportunity for the development of low-cost commercially available sensors for analysis of a number of analytes in the environment, e.g., customers by themselves can monitor toxic metals in their drinking water. Although, GPE based electrodes got a high degree of maturity from fabrication and modification point of view, but still they are at a significant distance from commercialization. The leading challenge, however, is to bring these GPE based electrochemical techniques in the hands of a common person without compromising accuracy and reliability. Indeed, technologies are needed that can help in the mass production of modified GPEs for reliable detection of certain analytes in environmental matrices. The miniaturized devices at mass level can only be generated by the integration between electronics, electrochemistry, electrical engineering and analytical chemistry.Abbreviations
| PSS/CnP/GPE | Polystyrenesulfonate/carbon nanopowders/pencil graphite electrode; |
| Ag/FeOOH/GPE | Silver/FeOOH/GPE |
| PtNPs/GPE | Platinum nanoparticle-modified GPE |
| Hb/PLE | Hemoglobin/modified pencil lead electrode |
| PVF+/MWCNTs/GPE | Poly(vinylferrocenium)/multi-walled carbon nanotubes/GPE |
| Bi-NPs/puMWCNT/GPE | Bismuth NPs/purified multiwalled carbon nanotubes/GPE |
| NG/PG/BiE | Nafion graphene/pencil graphite/bismuth film electrode |
| Poly(PyY)/GPE | Poly pyronin Y/pencil graphite electrode |
| Cu-P4R/PPy/GPE | Copper-Ponceau 4R azo dye/polypyrol/GPE |
| Au NPs/GPE | Gold nanoparticles/GPE |
| P4VP/GPE | Poly(4-vinyl pyridine)/GPE |
| DPC/PANI/GPE | Diphenylcarbazide/polyaniline/GPE |
| Cu–Car/PA/GPE | Carmoisine–Cu(II) complex/polyaniline/GPE |
| (HRP/AuNPs)2/CS/GPE | Horseradish peroxidase/gold nanoparticles/chitosan/GPE |
| Tar/PPy/GPE | Tartrazine/polypyrrole/GPE |
| pCu/GPE | Porous copper/graphite pencil electrode |
| ETGPEE | Electrochemically treated pencil graphite electrode |
| MIP/PPy/GPE | Molecular imprinted polymer/polypyrol/graphite pencil electrode |
| PGPE | Pretreated pencil graphite electrode |
| PANI/MWCNTs/GPE | Polyaniline nanorods/multiwalled carbon nanotubes/GPE |
| LOD | Limit of detection |
| AFS | Atomic fluorescence spectroscopy |
| FAAS | Flame atomic absorption spectroscopy |
| GFFAAS | Graphite furnace atomic absorption spectroscopy |
| ISE | Ion selective electrode |
| HRP-Au NPs/CCPE | Horseradish peroxidase-gold nanoparticles/chitosan carbon paste electrode |
| Pt–PdBNC/SPGFE | Pt–Pd bimetallic nanoclusters/screen-printed gold nanofilm electrode |
| AuNPs/AG/SPCE | Gold nanoparticles/activated graphite/screen printed carbon electrode |
Acknowledgements
The authors acknowledge the support provided by King Fahd University of Petroleum and Minerals for funding this work through project no. SB141010.References
- J. Mašek, J. Electroanal. Chem., 1960, 1, 416–421 Search PubMed
.
- A. Bund, J. Dittmann, D. Lordkipanidze and G. Schwitzgebel, Anal. Bioanal. Chem., 1996, 356, 27–30 CrossRef CAS PubMed
.
- L. Engel and W. Baumann, Fresenius. J. Anal. Chem., 1993, 346, 745–751 CrossRef CAS
.
- K. Aoki, T. Okamoto, H. Kaneko, K. Nozaki and A. Negishi, J. Electroanal. Chem. Interfacial Electrochem., 1989, 263, 323–331 CrossRef CAS
.
- A. M. Bond, P. J. Mahon, J. Schiewe and V. Vicente-Beckett, Anal. Chim. Acta, 1997, 345, 67–74 CrossRef CAS
.
- J. Wang, A.-N. Kawde and E. Sahlin, Analyst, 2000, 125, 5–7 RSC
.
- J. Wang and A.-N. Kawde, Anal. Chim. Acta, 2001, 431, 219–224 CrossRef CAS
.
- J. Wang, A.-N. Kawde, A. Erdem and M. Salazar, Analyst, 2001, 126, 2020–2024 RSC
.
- D. Demetriades, A. Economou and A. Voulgaropoulos, Anal. Chim. Acta, 2004, 519, 167–172 CrossRef CAS
.
- S. Teepoo, P. Chumsaeng, P. Nethan, W. Prueprang and P. Tumsae, Int. J. Electrochem. Sci., 2012, 7, 4645–4656 CAS
.
- I. G. David, I. A. Badea and G. L. Radu, Turk. J. Chem., 2013, 37, 91–100 CAS
.
- M. Abdul Aziz and A. N. Kawde, Talanta, 2013, 115, 214–221 CrossRef CAS PubMed
.
- M. Sajid, M. K. Nazal, M. Mansha, A. Alsharaa, S. M. S. Jillani and C. Basheer, TrAC, Trends Anal. Chem., 2016, 76, 15–29 CrossRef CAS
.
- P. B. Tchounwou, C. G. Yedjou, A. K. Patlolla and D. J. Sutton, EXS, 2012, 101, 133–164 Search PubMed
.
- J. L. Wee Ling, A. Khan, B. Saad and S. Ab Ghani, Talanta, 2012, 88, 477–483 CrossRef PubMed
.
- G. Flora, D. Gupta and A. Tiwari, Interdiscip. Toxicol., 2012, 5, 47–58 CAS
.
- H. Safardoust-Hojaghan, M. Salavati-Niasari, M. H. Motaghedifard and S. M. Hosseinpour-Mashkani, New J. Chem., 2015, 39, 4676–4684 RSC
.
- M.-K. Ali, R. Ansari, A. F. Delavar and Z. Mosayebzadeh, Bull. Korean Chem. Soc., 2012, 33, 1247–1252 CrossRef
.
- R. Ansari, A. F. Delavar and A. Mohammad-khah, J. Solid State Electrochem., 2012, 16, 3315–3322 CrossRef CAS
.
- K. Pokpas, N. Jahed, O. Tovide, P. G. Baker and E. I. Iwuoha, Int. J. Electrochem. Sci., 2014, 9, 5092–5115 CAS
.
- A. Cantalapiedra, M. J. Gismera, J. R. Procopio and M. T. Sevilla, Talanta, 2015, 139, 111–116 CrossRef CAS PubMed
.
- M. A. Z. Mosayebzadeh, R. Ansari and A. Mohammad-khah, Anal. Bioanal. Electrochem., 2013, 5, 109–129 Search PubMed
.
- R. Ansari, A. F. Delavar, A. Aliakbar and A. Mohammad-Khah, J. Solid State Electrochem., 2012, 16, 869–875 CrossRef CAS
.
- A. Mehdinia, S. Shegefti and F. Shemirani, Talanta, 2015, 144, 1266–1272 CrossRef CAS PubMed
.
- W.-C. Tseng, K.-C. Hsu, C. S. Shiea and Y.-L. Huang, Anal. Chim. Acta, 2015, 884, 1–18 CrossRef CAS PubMed
.
- L. Trzonkowska, B. Leśniewska and B. Godlewska-Żyłkiewicz, Crit. Rev. Anal. Chem., 2016, 46, 305–322 CrossRef CAS PubMed
.
- M. Sajid and C. Basheer, TrAC, Trends Anal. Chem., 2016, 75, 174–182 CrossRef CAS
.
- C. Karuppiah, S. Palanisamy, S. Chen, S. Kannan and P. Periakaruppan, Electrochim. Acta, 2014, 139, 157–164 CrossRef CAS
.
- B. E. Watt, A. T. Proudfoot and J. A. Vale, Toxicol. Rev., 2004, 23, 51–57 CrossRef CAS PubMed
.
- A.-N. Kawde, M. Aziz, N. Baig and Y. Temerk, J. Electroanal. Chem., 2015, 740, 68–74 CrossRef CAS
.
- M. A. Aziz and A. N. Kawde, Microchim. Acta, 2013, 180, 837–843 CrossRef
.
- J. Zhang and J. Zheng, Anal. Methods, 2015, 7, 1788–1793 RSC
.
- F. Kuralay, M. Dumangöz and S. Tunç, Talanta, 2015, 144, 1133–1138 CrossRef CAS PubMed
.
- K. Daǧci and M. Alanyalioǧlu, J. Electroanal. Chem., 2013, 711, 17–24 CrossRef
.
- M. Sajid, C. Basheer, K. Narasimhan, A. Buhmeida, A. Qahtani and M. S. Al-ahwal, Nat., Environ. Pollut. Technol., 2016, 15, 733–746 Search PubMed
.
- US EPA, Phenol|Technology Transf. Netw. Air Toxics Website|US EPA, http//www.epa.gov/ttnatw01/hlthef/phenol.html.
- A. Rana and A. N. Kawde, Electroanalysis, 2016, 28, 898–902 CrossRef CAS
.
- T. Galeano-Diaz, A. Guiberteau-Cabanillas, N. Mora-Diez, P. Parrilla-Vazquez and F. Salinas-Lopez, J. Agric. Food Chem., 2000, 48, 4508–4513 CrossRef CAS PubMed
.
- Y. Yang, B. Unnikrishnan and S. Chen, Scanning, 2011, 6, 3902–3912 CAS
.
- A.-N. Kawde and M. A. Aziz, Electroanalysis, 2014, 26, 2484–2490 CrossRef CAS
.
- K. Asadpour-Zeynali and P. Najafi-Marandi, Electroanalysis, 2011, 23, 2241–2247 CrossRef CAS
.
- C. A. Staples, P. B. Dom, G. M. Klecka, T. O. Sandra and L. R. Harris, Chemosphere, 1998, 36, 2149–2173 CrossRef CAS PubMed
.
- C. Erler and J. Novak, J. Pediatr. Nurs., 2010, 25, 400–407 CrossRef PubMed
.
- S. Poorahong, C. Thammakhet, P. Thavarungkul, W. Limbut, A. Numnuam and P. Kanatharana, Microchim. Acta, 2012, 176, 91–99 CrossRef CAS
.
- A. Özcan, Electroanalysis, 2014, 26, 1631–1639 CrossRef
.
- B. D. C. Ventura-camargo and M. A. Marin-morales, Textiles and Light Industrial Science and Technology, 2013, 2, 85–103 Search PubMed
.
- A. A. Ensafi, H. R. Jamei, E. Heydari-Bafrooei and B. Rezaei, Sens. Actuators, B, 2014, 202, 224–231 CrossRef CAS
.
- K. K. Tadi, R. V. Motghare and V. Ganesh, Electroanalysis, 2014, 26, 2328–2336 CrossRef CAS
.
- A. Ensafi, B. Rezaei, M. Amini and E. Heydari-Bafrooei, Talanta, 2012, 88, 244–251 CrossRef CAS PubMed
.
- M. W. Aktar, D. Sengupta and A. Chowdhury, Interdiscip. Toxicol., 2009, 2, 1–12 CrossRef PubMed
.
- Z. O. Uygun and Y. Dilgin, Sens. Actuators, B, 2013, 188, 78–84 CrossRef CAS
.
- Y. Yardim, E. Keskin, A. Levent, M. Ozsöz and Z. Sentürk, Talanta, 2010, 80, 1347–1355 CrossRef CAS PubMed
.
- E. Keskin, Y. Yardım and Z. Şentürk, Electroanalysis, 2010, 22, 1191–1199 CrossRef CAS
.
- C. Gao, Z. Guo, J.-H. Liu and X.-J. Huang, Nanoscale, 2012, 4, 1948–1963 RSC
.
- A. J. Saleh Ahammad, J. Lee and M. Rahman, Sensors, 2009, 9, 2289–2319 CrossRef CAS PubMed
.
- E. Alipour, M. R. Majidi, A. Saadatirad, S. M. Golabi and A. M. Alizadeh, Electrochim. Acta, 2013, 91, 36–42 CrossRef CAS
.
- E. Alipour and S. Gasemlou, Anal. Methods, 2012, 4, 2962 RSC
.
- N. Baig and A.-N. Kawde, RSC Adv., 2016, 6, 80756–80765 RSC
.
- S. Yuan, W. Chen and S. Hu, Talanta, 2004, 64, 922–928 CrossRef CAS PubMed
.
- W. Zhao, H. Wang, X. Qin, X. Wang, Z. Zhao, Z. Miao, L. Chen, M. Shan, Y. Fang and Q. Chen, Talanta, 2009, 80, 1029–1033 CrossRef CAS PubMed
.
- Y. Liu, R. Yuan, Y. Chai, D. Tang, J. Dai and X. Zhong, Sens. Actuators, B, 2006, 115, 109–115 CrossRef CAS
.
- M. Pravda, C. O. Meara and G. G. Guilbault, Talanta, 2001, 54, 887–892 CrossRef CAS PubMed
.
- W. Yantasee, Y. Lin, G. E. Fryxell and B. J. Busche, Anal. Chim. Acta, 2004, 502, 207–212 CrossRef CAS
.
- A. Rana and A. Kawde, J. Chin. Chem. Soc., 2016, 63, 668–676 CrossRef CAS
.
- J. Wang, X. Chen, K. Liao, G. Wang and M. Han, Nanoscale Res. Lett., 2015, 1–6 Search PubMed
.
- C. Lei, S. Hu, G. Shen and R. Yu, Talanta, 2003, 59, 981–988 CrossRef CAS PubMed
.
- X. Niu, C. Chen, H. Zhao, Y. Chai and M. Lan, Biosens. Bioelectron., 2012, 36, 262–266 CrossRef CAS PubMed
.
- Ö. Sağlam, D. G. Dilgin, B. Ertek and Y. Dilgin, Mater. Sci. Eng., C, 2016, 60, 156–162 CrossRef PubMed
.
- S. Pourbeyram and K. Mehdizadeh, J. Food Drug Anal., 2016, 1–9 Search PubMed
.
- I. G. David, D. E. Popa, M. Buleandra, Z. Moldovan, E. E. Iorgulescu and I. A. Badea, Anal. Methods, 2016, 8, 6537–6544 RSC
.
- A. Erdem, P. Papakonstantinou and H. Murphy, Anal. Chem., 2006, 78, 6656–6659 CrossRef CAS PubMed
.
- N. Baig and A.-N. Kawde, Anal. Methods, 2015, 7, 9535–9541 RSC
.
- A. Alsharaa, C. Basheer and M. Sajid, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, 1007, 43–48 CrossRef CAS PubMed
.
- M. Sajid, C. Basheer, A. Alsharaa, K. Narasimhan, A. Buhmeida, M. Al Qahtani and M. S. Al-Ahwal, Anal. Chim. Acta, 2016, 924, 35–44 CrossRef CAS PubMed
.
- M. Sajid and C. Basheer, J. Chromatogr. A, 2016, 1455, 37–44 CrossRef CAS PubMed
.
- M. Sajid, A.-N. Kawde and M. Daud, J. Saudi Chem. Soc., 2015, 19, 689–705 CrossRef
.
- J. Zhang, J. Fang and X. Duan, Spectrochim. Acta, Part B, 2016, 122, 52–55 CrossRef CAS
.
- A. Mirabi, Z. Dalirandeh and A. S. Rad, J. Magn. Magn. Mater., 2015, 381, 138–144 CrossRef CAS
.
- F. Omidi, M. Behbahani, M. Kalate Bojdi and S. J. Shahtaheri, J. Magn. Magn. Mater., 2015, 395, 213–220 CrossRef CAS
.
- M. Fayazi, M. A. Taher, D. Afzali, A. Mostafavi and M. Ghanei-Motlagh, Mater. Sci. Eng., C, 2016, 60, 365–373 CrossRef CAS PubMed
.
- G. Fakhriyan, H. Z. Mousavi and S. M. Sajjadi, Anal. Methods, 2016, 8, 995–1002 RSC
.
- E. Yilmaz and M. Soylak, J. Anal. At. Spectrom., 2015, 30, 1629–1635 RSC
.
- S. M. Sorouraddin and S. Nouri, Anal. Methods, 2016, 8, 1396–1404 RSC
.
- E. Kendüzler and A. R. Türker, Anal. Sci., 2002, 18, 917–921 CrossRef
.
- K. Shrivas, K. Dewangan and A. Ahmed, Anal. Methods, 2016, 8, 5519–5525 RSC
.
- A. Levent, E. Keskin, Y. Yardım and Z. Şentürk, Rev. Anal. Chem., 2011, 30, 45–51 CAS
.
- T. Thomas, R. J. Mascarenhas, O. J. D. Souza, P. Martis, J. Dalhalle and B. E. K. Swamy, J. Colloid Interface Sci., 2013, 402, 223–229 CrossRef CAS PubMed
.
- L. Zhang, Y. Li, L. Zhang, D. Li, D. Karpuzov and Y. Long, Int. J. Electrochem. Sci., 2011, 6, 819–829 CAS
.
- J. Wang, M. Li, Z. Shi, N. Li and Z. Gu, Microchem. J., 2002, 73, 325–333 CrossRef CAS
.
- M. Pumera and S. Alegret, Electrophoresis, 2007, 28, 1274–1280 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |




