DOI:
10.1039/C6RA17425F
(Paper)
RSC Adv., 2016,
6, 79578-79583
Formation of mesoporous calcium sulfate microspheres through phase conversion in controlled calcination†
Received
7th July 2016
, Accepted 16th August 2016
First published on 17th August 2016
Abstract
Calcium sulfate has been used as a biomedical material for over a century thanks to its biocompatible, biodegradable and cementitious properties. However the dense structure and low surface area of existing materials exclude its adsorption properties and restrict the applications. In this work, mesoporous calcium sulfate microspheres are prepared through topological conversion of monodisperse α-calcium sulfate hemihydrate (α-HH) microspheres under controlled calcination. The calcination process contributes to the phase conversion from α-HH to calcium sulfate anhydrite (AH) along with the elimination of structural water and the reduction of crystallite size, resulting in the formation of mesopores. The microspheres composed of 29.56 wt% α-HH and 70.44 wt% AH demonstrate a maximum surface area of 30.62 m2 g−1 and an ibuprofen (IBU) loading capacity of 22.44 mg g−1, which shows a one hundred times improvement in surface area compared with traditional calcium sulfate materials. This is the first time drugs have been loaded directly to calcium sulfate particles, showing that the mesoporous calcium sulfate microspheres with uniform particle size and ordered pore structure could be a promising carrier as a biomaterial.
Introduction
Mesoporous materials are of great interest for a wide range of applications due to their morphology/structure dependent optical, magnetic, electronic, catalytic and biomedical properties.1–5 Calcium sulfate, composed of only Ca2+ and SO42− ions and water molecules (CaSO4·xH2O, x = 0, 0.5 and 2.0) has been considered as a strong candidate compound for biomedical use due to its excellent workability, biocompatibility, biodegradability and osteoconductivity.6–8 Therein, calcium sulfate hemihydrate (HH), particularly α-HH, possesses a well-known cementitious property for bone repair, and is regarded as the most important phase of calcium sulfate.9,10 Up to now, α-HH particles usually present in micro size with shapes of columns, rods, wires and whiskers.11–13 Such a monotonous and dense structure has a surface area as low as only 0.18–0.31 m2 g−1, restraining its use as a carrier for drugs or growth factors which are essential for bone regeneration in the biomedical field.14 Methods have been developed to blend α-HH with other mesoporous materials such as non-resorbable polymethylmethacrylate (PMMA) and apatite to endow the composites with adsorption properties. However, the practical problem is that additional surgery has to be conducted for the removal of non-biodegradable materials or a side effect arises from the remarkable weakening of the cementitious property of α-HH.6,15,16 Therefore, an understanding of calcium sulfate particles acting as drug carriers is desirable.17
Strategies like adjusting pH, electrolyte concentration or adding crystal modifier have been applied to obtain different morphologies of α-HH.18,19 However, only the aspect ratio can be controlled, leaving a big challenge to regulate crystal structure or create pores. Recently, our group has developed a facile method to control the morphology and structure of α-HH, successfully synthesizing α-HH polycrystals in the shape of microsphere and ellipsoid.20 Here, we develop the monodisperse α-HH microspheres into mesoporous calcium sulfate microspheres through topological conversion in the hope of improving its surface area and adsorption capacity by controlled calcination. The phase composition is regulated to gain superior pore property for drug loading and keep the spherical outline. With the mesoporous structure and suitable drug loading capacity, the calcium sulfate microspheres, a relatively abundant mineral will find its way in the field of biomedicine as an inexpensive and biocompatible carrier.
Results and discussion
Morphology and phase control under calcination
Elevated temperatures are always used in phase conversion and modification of materials. The morphologies of as-formed α-HH and its products calcined at different temperatures are presented in Fig. 1 and S1.† The monodisperse α-HH microspheres with smooth surface are formed by self-assembly of nanoparticles in length of 30–100 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and demonstrate a diameter of ∼2 μm (Fig. 1a). The calcination of α-HH microspheres at lower temperature of 200 °C results in the formation of small pores on the surface with a random distribution (Fig. 1b), and the pores increase greatly in number at 300 °C (Fig. 1c). When the temperature reaches 400 °C, the pores distribute uniformly over the microspheres (Fig. 1d). As increasing temperature to 500 and 600 °C, the surface of microspheres consists of more pores in irregular shape with expanding gaps (Fig. 1e and f). At a high temperature of 700 °C, the nanoparticles which compose the microspheres seem to be melted and reshaped into larger particles with a length of ∼500 nm and a width of ∼300 nm, leading to a walnut-like morphology (Fig. 1g). When the temperature is raised to 800 °C, the framework of microspheres collapses and the irregular particles with a size of 200–700 nm stack compactly (Fig. 1h). The pores of microspheres grow denser and larger with the increase of calcination temperature, and the high temperature above 700 °C leads to the collapse of framework and the elimination of pores. The average particle size of microspheres calcined at temperatures lower than 600 °C keeps almost the same with that of as-formed α-HH microspheres (∼2 μm), while it decreases to ∼1.82 μm at 700 °C and ∼1.64 μm at 800 °C (Fig. S2†).
20 and demonstrate a diameter of ∼2 μm (Fig. 1a). The calcination of α-HH microspheres at lower temperature of 200 °C results in the formation of small pores on the surface with a random distribution (Fig. 1b), and the pores increase greatly in number at 300 °C (Fig. 1c). When the temperature reaches 400 °C, the pores distribute uniformly over the microspheres (Fig. 1d). As increasing temperature to 500 and 600 °C, the surface of microspheres consists of more pores in irregular shape with expanding gaps (Fig. 1e and f). At a high temperature of 700 °C, the nanoparticles which compose the microspheres seem to be melted and reshaped into larger particles with a length of ∼500 nm and a width of ∼300 nm, leading to a walnut-like morphology (Fig. 1g). When the temperature is raised to 800 °C, the framework of microspheres collapses and the irregular particles with a size of 200–700 nm stack compactly (Fig. 1h). The pores of microspheres grow denser and larger with the increase of calcination temperature, and the high temperature above 700 °C leads to the collapse of framework and the elimination of pores. The average particle size of microspheres calcined at temperatures lower than 600 °C keeps almost the same with that of as-formed α-HH microspheres (∼2 μm), while it decreases to ∼1.82 μm at 700 °C and ∼1.64 μm at 800 °C (Fig. S2†).
 |
| | Fig. 1 High magnification FESEM images of as-formed α-HH microspheres (a) and microspheres calcined at temperatures of (b) 200 °C, (c) 300 °C, (d) 400 °C, (e) 500 °C, (f) 600 °C, (g) 700 °C and (h) 800 °C in air for 180 s. | |
The as-formed microspheres consist of pure α-HH phase.20 The phase evolution of calcined microspheres is examined by XRD and TG analysis. After calcination at a low temperature of 200 and 300 °C, the intensity of characteristic peaks for α-HH at 14.69°, 29.70° and 31.89° decreases (Fig. 2), while several new peaks for soluble anhydrite (AIII-CaSO4) at 25.54° and 32.88° appear, indicating part of α-HH crystallites transform to AIII-CaSO4.21 The fraction of AIII-CaSO4 is about 5.48 wt% and 10.79 wt% respectively at 200 and 300 °C (Table 1), denoted by the weight losses of −5.87 wt% and −5.54 wt% compared with −6.21 wt% of α-HH occurring at 35–200 °C (Fig. S3†). When the temperature reaches 400 °C, the microsphere is composed of 55.39 wt% α-HH and 44.61 wt% insoluble anhydrite (AII-CaSO4), recognized by the diagnostic peaks at 22.91°, 25.44°, 29.70°, 31.37° and 31.89°. The fraction of AII-CaSO4 grows rapidly to 84.06 wt% at 500 °C and 85.51 wt% at 600 °C, while a weak peak at 29.70° indicates the presence of trace α-HH. Upon a further increase of temperature to 700 and 800 °C, AII-CaSO4 becomes the unique phase in the microspheres. Evidently, α-HH transforms gradually to AIII-CaSO4 and then AII-CaSO4 with the increase of calcination temperature, and AII-CaSO4 becomes the dominant phase at higher temperature. The peaks at 25.44° and 31.37° become stronger from 400 to 800 °C, implying the enhancement in crystallinity of AII-CaSO4 crystallites.
 |
| | Fig. 2 XRD patterns of as-formed α-HH microspheres and microspheres calcined at temperatures from 200 to 800 °C in air for 180 s. HH is denoted by the characteristic peaks at 14.69°, 25.62°, 29.70°, 31.89° and 32.98° (PDF 47-0964). The diagnostic peaks at 14.64°, 25.54°, 29.60° and 32.88° are assigned to AIII-CaSO4 (PDF 45-0157) and AII-CaSO4 is recognized by peaks at 22.91°, 25.44°, 28.58°, 31.37° and 31.97° (PDF 37-1496). | |
Table 1 Phase composition, pore volume (Vp), average pore size (Dp) and BET surface area (SBET) of as-formed α-HH microspheres and microspheres calcined at temperatures from 200 to 800 °C in air for 180 s
| T (°C) |
Phase composition (wt%) |
Vp (cm3 g−1) |
Dp (nm) |
SBET (m2 g−1) |
| — |
α-HH |
0.008 |
12.0 |
2.91 |
| 200 |
α-HH (94.52) + AIII-CaSO4 (5.48) |
0.024 |
12.6 |
8.67 |
| 300 |
α-HH (89.21) + AIII-CaSO4 (10.79) |
0.028 |
13.7 |
10.09 |
| 400 |
α-HH (55.39) + AII-CaSO4 (44.61) |
0.069 |
17.6 |
18.30 |
| 500 |
α-HH (15.94) + AII-CaSO4 (84.06) |
0.076 |
25.4 |
15.74 |
| 600 |
α-HH (14.49) + AII-CaSO4 (85.51) |
0.079 |
27.0 |
14.37 |
| 700 |
AII-CaSO4 |
0.005 |
8.1 |
2.81 |
| 800 |
AII-CaSO4 |
0.002 |
2.8 |
2.14 |
Evolution of pore property and crystallite size
Nitrogen adsorption–desorption isotherms were measured to characterize the porosity and test Brunauer–Emmett–Teller (BET) surface area of the microspheres. The microspheres calcined at 200, 400 and 600 °C all demonstrate type-IV isotherms with typical type H3 hysteresis loops over the range of 0.4 < P/P0 < 1.0, indicating the presence of mesopores and macropores (Fig. 3a).22,23 The isotherm of microspheres calcined at 400 °C has the largest hysteresis loop, suggesting the maximum volume of interior mesopores.24 The profiles of pore size distribution in Fig. 3b illustrate that the as-formed α-HH microspheres are gifted a non-porous structure, which is also proved by FESEM image (Fig. 1a). The pore size distribution profile of microspheres calcined at 200 °C presents in a gentle slope, and then the slope changes abruptly into a clear and sharp peak at 400 °C, indicating the formation of ordered mesoporous structure. However the peak size decreases at 600 °C, implying the emerge of macropores caused by the growth of AII-CaSO4 crystallites.25 The peak disappears as the temperature increases to 800 °C, according with the framework collapse of microspheres and the non-porous structure observed in Fig. 1h.
 |
| | Fig. 3 Nitrogen adsorption–desorption isotherms (a) and pore size distributions (b) of as-formed α-HH microspheres and microspheres calcined at temperatures from 200 to 800 °C in air for 180 s. | |
The pore volume, average pore size and BET surface area show a similar evolution tendency (Table 1), increasing and then decreasing after a maximum value as the calcination temperature increases. The pore volume increases with the increment of AH fraction, indicating the elimination of crystal water is responsible for the formation of mesopores. Upon the complete conversion of α-HH to AII-CaSO4 at 700 °C, the pore volume decreases to 0.005 cm3 g−1, even lower than 0.008 cm3 g−1 of as-formed α-HH microspheres. It is noted that the pore volume of calcined microspheres increases slightly from 400 to 600 °C, yet the surface area achieves higher value of 18.30 cm2 g−1 at 400 °C. Thanks to the smallest average pore size of 17.6 nm and the most uniform pore size distribution (Fig. 3b). The calcination leads to microstructural variation due to the fusion and reconstruction of the crystallites,26 while displaying on the other side is the morphology and porous structure evolution.
Williamson–Hall (W–H) plot analysis27 is performed to investigate the variation of average crystallite size and micro-strain presenting in as-formed and calcined microspheres. The W–H equation includes the size- and strain-induced broadening as a function of 2θ.
(βobs − βinst)cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = kλ/D + 4ε θ = kλ/D + 4ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
where
βobs and
βinst are observed and instrumental integral peak breadth in radian 2
θ, respectively;
ε is the weighted average micro-strain associated with the crystallites; and
D is the volume weighted crystallite size. As shown in
Fig. 4, the micro-strain grows continuously with calcination temperature increasing, suggesting the increase in the degree of interior disorder and defects which probably arise from rapid cooling to room temperature after calcination.
28 The average crystallite size of α-HH microspheres is 50.6 nm, while that of calcined microspheres decreases slightly at 200 and 300 °C, to 44.8 nm at 400 °C as the elimination of structural water in phase conversion from α-HH to AH. The rapid crystalline growth of AII-CaSO
4 accounts for the subsequent increment of crystallite size to 92.1 nm at 800 °C.
29 The part conversion of α-HH to AH contributes to the increase of pore volume derived from the reduction of crystallite size, while the subsequent growth of AII-CaSO
4 crystallites deteriorates the degree of interior order and pore size distribution.
30 Thus an optimal calcination condition exists for the maximum surface area.
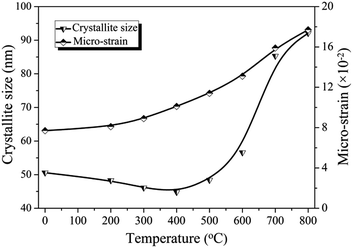 |
| | Fig. 4 Crystallite size and micro-strain of as-formed α-HH microspheres and microspheres calcined at temperatures from 200 to 800 °C in air for 180 s. | |
Optimization of surface area and IBU loading
The synergetic effect of calcination temperature and time on the surface area of calcium sulfate microspheres is examined in Fig. 5 and Table S1.† The surface area increases gradually and decreases subsequently after a maximum value with calcination time extension at a fixed temperature. It takes less calcination time to obtain peak value of surface area of microspheres at a higher temperature. The time needed at 800 °C is 20 s, which is twelfth of that at 400 °C (Table 2). The microspheres calcined under different temperatures with peak value of surface area share almost the same phase composition of 30–35 wt% α-HH and 65–70 wt% AII-CaSO4. Such a phase composition favors a better porosity and avoids the collapse of spherical framework. The microspheres calcined at 500 °C for 120 s (A500) achieves the highest surface are of 30.62 m2 g−1 resulted from the highest pore volume and smallest average pore size. The mesopores distribute uniformly on the surface of A500 shown in Fig. 6a. A500 displays a typical type-IV isotherm and a narrow pore size distribution profile with a peak at 12.1 nm (Fig. 6b), which accounts for the increase of surface area up to ten times higher than that of α-HH microspheres.
 |
| | Fig. 5 BET surface areas of microspheres calcined at temperatures from 400 to 800 °C for different times from 10 to 300 s in air. | |
Table 2 Calcination time, AII-CaSO4 fraction, pore volume (Vp), average pore size (Dp), BET surface area (SBET) and loading capacity (LC) of microspheres calcined at 400–800 °C in air to gain the peak value of surface area
| No. |
T (°C) |
Time (s) |
AII-CaSO4 (wt%) |
Vp (cm3 g−1) |
Dp (nm) |
SBET (m2 g−1) |
LC (mg g−1) |
| A400 |
400 |
240 |
68.58 |
0.102 |
19.3 |
22.70 |
16.15 |
| A500 |
500 |
120 |
70.44 |
0.121 |
12.1 |
30.62 |
22.44 |
| A600 |
600 |
60 |
69.04 |
0.118 |
24.6 |
23.71 |
18.21 |
| A700 |
700 |
30 |
67.12 |
0.957 |
19.9 |
19.26 |
13.50 |
| A800 |
800 |
20 |
65.13 |
0.928 |
19.8 |
18.18 |
11.87 |
 |
| | Fig. 6 FESEM image of microspheres calcined at 500 °C for 120 s (A500) (a), nitrogen adsorption–desorption isotherm and pore size distribution (inset) of A500 (b), IBU loading capacity of as-formed α-HH microspheres and A500 (c), and FTIR spectra of (1) A500-IBU, (2) A500 and (3) IBU (d). The characteristic peaks at 1621, 1117 and 1008 cm−1 are attributed to α-HH and the peaks at 1157, 676 and 595 cm−1 are assigned to AH. The diagnostic peaks at 2955, 2924, 2869, 1708, 1463 and 1421 cm−1 belong to IBU. | |
The loading capacity of as-formed α-HH microspheres and calcined microspheres (A500) both increases linearly at IBU concentrations of 0.25–1.00 mg ml−1 and then keeps constant when IBU concentration is above 1.0 mg ml−1 (Fig. 6c). The loading capacity of A500 is 22.44 mg g−1, which is nine times higher than 2.40 mg g−1 of as-formed α-HH microspheres. The diagnostic peaks at 1621 cm−1 (γ2 H2O vibration), 1157 cm−1 (γ3 SO42− vibration), 1117 cm−1 (γ3 SO42− vibration), 1008 cm−1 (γ1 SO42− vibration), 676 and 595 cm−1 (γ4 SO42− vibration) in FTIR spectra of A500-IBU (Fig. 6d-(1)) demonstrate the presence of calcium sulfate, which are consistent with those of A500 (Fig. 6d-(2)). The characteristic peaks at 2955 and 2869 cm−1 (–CH3 vibration) and 1708 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration)31 indicate IBU loading into A500 (Fig. 6d-(1)), while verified by the peaks of IBU (Fig. 6d-(3)). The loading capacity of A400, A600, A700 and A800 is 16.15, 22.44, 18.21, 13.50 and 11.87 mg g−1 respectively (Table 2), showing a correlation factor of 0.97 with the surface area. Since IBU is a micromolecular drug,32 its loading capacity probably depends on the surface area instead of the pore size.33
O vibration)31 indicate IBU loading into A500 (Fig. 6d-(1)), while verified by the peaks of IBU (Fig. 6d-(3)). The loading capacity of A400, A600, A700 and A800 is 16.15, 22.44, 18.21, 13.50 and 11.87 mg g−1 respectively (Table 2), showing a correlation factor of 0.97 with the surface area. Since IBU is a micromolecular drug,32 its loading capacity probably depends on the surface area instead of the pore size.33
Mechanism
A schematic illustration for microstructural evolution, phase conversion and corresponding surface area change is shown in Fig. 7. The as-formed α-HH microspheres with smooth surface have a dense structure, showing a low surface area (2.91 m2 g−1) and poor loading capacity (Fig. 7a). As α-HH partly converts to AH phase through calcination, the mesopores starts to distribute in the microspheres along with the elimination of crystal water (Fig. 7b). During the phase conversion, the gradually decreasing crystallite size (Fig. 4) contributes to the increasing of porosity and the narrowing of pore size distribution, thus increases the surface area (Table 1). A desired phase composition of 29.56 wt% α-HH and 70.44 wt% AH creates the highest surface area (30.62 m2 g−1), nine times more than that of as-formed microspheres (Fig. 7c). In comparison, the exceeding calcination degree, for example, exorbitant temperature or overlong time will deteriorate the microstructure of the microspheres (Fig. 7d). The reconstruction and growth of AH crystallites fuse part of mesopores, leaving behind a broaden pore size distribution (Fig. 3b). This process contributes to the crystallinity improvement of AH crystallites (Fig. 2), but remarkably reduces the surface area (Table 1). The further crystalline growth finally leads to the framework collapse and total destruction of the pore structure (Fig. 7e).34 The phase conversion and crystalline growth compete with each other to determine the pore volume and pore size distribution, thus an optimal calcination time exists at a certain temperature (Fig. 5). The crystalline growth of AH is more severe at high temperature, which is liable to increase the degree of interior disorder (Fig. 4) and worsen the pore size distribution.35 Therefore, a moderate temperature of 500 °C is beneficial for the formation of microspheres with uniform and ordered pore structure. Such a controllable calcination offers the mesoporous calcium sulfate microspheres a satisfactory loading capacity, which will provide more opportunities for its applications in biomedical field.
 |
| | Fig. 7 Schematic illustration of evolution in microstructure, phase composition and surface area of calcium sulfate microspheres during the calcination process. | |
Conclusions
The controlled calcination of α-HH microspheres is subjected to prepare the mesoporous calcium sulfate microspheres. The elimination of structural water and the reduction of crystallite size during phase conversion benefit the increase of porosity, while the subsequent crystalline growth deteriorates the interior order and the pore size distribution. By the regulation of calcination time and temperature, the phase composition is optimized at 30–35 wt% α-HH and 65–70 wt% AII-CaSO4, which balances the porosity and the spherical framework. The microspheres calcined at 500 °C for 120 s possess the surface area 100 times higher than those of traditional calcium sulfate materials. This work paves a way to create mesopores and improve surface area of calcium sulfate through controllable phase conversion, which is readily extended to other mineral materials.
Acknowledgements
We thank the National Science Foundation of China (Project 21176219) for support of this research.
Notes and references
- X. Zhang, J. Bai, B. Yang, G. Li and L. Liu, RSC Adv., 2016, 6, 58925–58932 RSC.
- M. Nandi, J. Mondal, K. Sarkar, Y. Yamauchi and A. Bhaumik, Chem. Commun., 2011, 47, 6677–6679 RSC.
- K. C. W. Wu, Y. Yamauchi, C. Y. Hong, Y. H. Yang, Y. H. Liang, T. Funatsu and M. Tsunoda, Chem. Commun., 2011, 47, 5232–5234 RSC.
- Y. Yamauchi, N. Suzuki, L. Radhakrishnan and L. Wang, Chem. Rec., 2009, 9, 321–339 CrossRef CAS PubMed.
- S. Zhan, D. Zhu, M. Qiu, H. Yu and Y. Li, RSC Adv., 2015, 5, 29353–29361 RSC.
- J. Doadrio, D. Arcos, M. Cabanas and M. Vallet-Regı, Biomaterials, 2004, 25, 2629–2635 CrossRef CAS PubMed.
- H. M. Jung, G. A. Song, Y. K. Lee, J. H. Baek, H. M. Ryoo, G. S. Kim, P. H. Choung and K. M. Woo, Biomaterials, 2010, 31, 29–37 CrossRef CAS PubMed.
- M. A. Rauschmann, T. A. Wichelhaus, V. Stirnal, E. Dingeldein, L. Zichner, R. Schnettler and V. Alt, Biomaterials, 2005, 26, 2677–2684 CrossRef CAS PubMed.
- S. Sato, T. Koshino and T. Saito, Biomaterials, 1998, 19, 1895–1900 CrossRef CAS PubMed.
- M. Cabanas, L. Rodriguez-Lorenzo and M. Vallet-Regi, Chem. Mater., 2002, 14, 3550–3555 CrossRef CAS.
- B. Guan, L. Yang, Z. Wu, Z. Shen, X. Ma and Q. Ye, Fuel, 2009, 88, 1286–1293 CrossRef CAS.
- Y. W. Wang, Y. Y. Kim, H. K. Christenson and F. C. Meldrum, Chem. Commun., 2012, 48, 504–506 RSC.
- Y. W. Wang and F. C. Meldrum, J. Mater. Chem., 2012, 22, 22055–22062 RSC.
- M. Tang, X. Shen and H. Huang, Mater. Sci. Eng., C, 2010, 30, 1107–1111 CrossRef CAS.
- T. Tuzuner, I. Sencan, D. Ozdemir, M. Alper, S. Duman, T. Yavuz and M. Yildirim, J. Chemother., 2006, 18, 628–633 CrossRef CAS PubMed.
- H. A. Doty, M. R. Leedy, H. S. Courtney, W. O. Haggard and J. D. Bumgardner, J. Mater. Sci.: Mater. Med., 2014, 25, 1449–1459 CrossRef CAS PubMed.
- M. V. Thomas and D. A. Puleo, J. Biomed. Mater. Res., Part B, 2009, 88, 597–610 CrossRef PubMed.
- X. Song, S. Sun, W. Fan and H. Yu, J. Mater. Chem., 2003, 13, 1817 RSC.
- B. Kong, B. Guan, M. Z. Yates and Z. Wu, Langmuir, 2012, 28, 14137–14142 CrossRef CAS PubMed.
- Q. Chen, G. Jiang, C. Jia, H. Wang and B. Guan, CrystEngComm, 2015, 17, 8549–8554 RSC.
- A. N. Christensen, M. Olesen, Y. Cerenius and T. R. Jensen, Chem. Mater., 2008, 20, 2124–2132 CrossRef CAS.
- H. Hou, L. Wang, F. Gao, G. Wei, B. Tang, W. Yang and T. Wu, J. Am. Chem. Soc., 2014, 136, 16716–16719 CrossRef CAS PubMed.
- J. Y. Cho, W. H. Nam, Y. S. Lim, W. S. Seo, H. H. Park and J. Y. Lee, RSC Adv., 2012, 2, 2449–2453 RSC.
- W. Q. Wang, J. G. Wang, P. C. Sun, D. T. Ding and T. H. Chen, J. Colloid Interface Sci., 2009, 331, 156–162 CrossRef CAS PubMed.
- T. Mokkelbost, I. Kaus, T. Grande and M. A. Einarsrud, Chem. Mater., 2004, 16, 5489–5494 CrossRef CAS.
- Q. Bao, J. Li, C. M. Li, Z. L. Dong, Z. Lu, F. Qin, C. Gong and J. Guo, J. Phys. Chem. B, 2008, 112, 12270–12278 CrossRef CAS PubMed.
- G. Williamson and W. Hall, Acta Metall., 1953, 1, 22–31 CrossRef CAS.
- J. G. Yu, H. G. Yu, B. Cheng, X. J. Zhao, J. C. Yu and W. K. Ho, J. Phys. Chem. B, 2003, 107, 13871–13879 CrossRef CAS.
- L. Guo, H. Hagiwara, S. Ida, T. Daio and T. Ishihara, ACS Appl. Mater. Interfaces, 2013, 5, 11080–11086 CAS.
- H. Tüysüz, C. Weidenthaler, T. Grewe, E. L. Salabaş, M. J. Benitez Romero and F. Schüth, Inorg. Chem., 2012, 51, 11745–11752 CrossRef PubMed.
- S. W. Song, K. Hidajat and S. Kawi, Langmuir, 2005, 21, 9568–9575 CrossRef CAS PubMed.
- M. Vallet-Regi, A. Rámila, R. P. del Real and J. Pérez-Pariente, Chem. Mater., 2001, 13, 308–311 CrossRef CAS.
- M. C. Kimling and R. A. Caruso, J. Mater. Chem., 2012, 22, 4073–4082 RSC.
- B. Lee, D. Lu, J. N. Kondo and K. Domen, Chem. Commun., 2001, 2118–2119 RSC.
- A. T. Vu, S. Jiang, Y. H. Kim and C. H. Lee, Ind. Eng. Chem. Res., 2014, 53, 13228–13235 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17425f |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and demonstrate a diameter of ∼2 μm (Fig. 1a). The calcination of α-HH microspheres at lower temperature of 200 °C results in the formation of small pores on the surface with a random distribution (Fig. 1b), and the pores increase greatly in number at 300 °C (Fig. 1c). When the temperature reaches 400 °C, the pores distribute uniformly over the microspheres (Fig. 1d). As increasing temperature to 500 and 600 °C, the surface of microspheres consists of more pores in irregular shape with expanding gaps (Fig. 1e and f). At a high temperature of 700 °C, the nanoparticles which compose the microspheres seem to be melted and reshaped into larger particles with a length of ∼500 nm and a width of ∼300 nm, leading to a walnut-like morphology (Fig. 1g). When the temperature is raised to 800 °C, the framework of microspheres collapses and the irregular particles with a size of 200–700 nm stack compactly (Fig. 1h). The pores of microspheres grow denser and larger with the increase of calcination temperature, and the high temperature above 700 °C leads to the collapse of framework and the elimination of pores. The average particle size of microspheres calcined at temperatures lower than 600 °C keeps almost the same with that of as-formed α-HH microspheres (∼2 μm), while it decreases to ∼1.82 μm at 700 °C and ∼1.64 μm at 800 °C (Fig. S2†).
20 and demonstrate a diameter of ∼2 μm (Fig. 1a). The calcination of α-HH microspheres at lower temperature of 200 °C results in the formation of small pores on the surface with a random distribution (Fig. 1b), and the pores increase greatly in number at 300 °C (Fig. 1c). When the temperature reaches 400 °C, the pores distribute uniformly over the microspheres (Fig. 1d). As increasing temperature to 500 and 600 °C, the surface of microspheres consists of more pores in irregular shape with expanding gaps (Fig. 1e and f). At a high temperature of 700 °C, the nanoparticles which compose the microspheres seem to be melted and reshaped into larger particles with a length of ∼500 nm and a width of ∼300 nm, leading to a walnut-like morphology (Fig. 1g). When the temperature is raised to 800 °C, the framework of microspheres collapses and the irregular particles with a size of 200–700 nm stack compactly (Fig. 1h). The pores of microspheres grow denser and larger with the increase of calcination temperature, and the high temperature above 700 °C leads to the collapse of framework and the elimination of pores. The average particle size of microspheres calcined at temperatures lower than 600 °C keeps almost the same with that of as-formed α-HH microspheres (∼2 μm), while it decreases to ∼1.82 μm at 700 °C and ∼1.64 μm at 800 °C (Fig. S2†).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = kλ/D + 4ε
θ = kλ/D + 4ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ
θ

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration)31 indicate IBU loading into A500 (Fig. 6d-(1)), while verified by the peaks of IBU (Fig. 6d-(3)). The loading capacity of A400, A600, A700 and A800 is 16.15, 22.44, 18.21, 13.50 and 11.87 mg g−1 respectively (Table 2), showing a correlation factor of 0.97 with the surface area. Since IBU is a micromolecular drug,32 its loading capacity probably depends on the surface area instead of the pore size.33
O vibration)31 indicate IBU loading into A500 (Fig. 6d-(1)), while verified by the peaks of IBU (Fig. 6d-(3)). The loading capacity of A400, A600, A700 and A800 is 16.15, 22.44, 18.21, 13.50 and 11.87 mg g−1 respectively (Table 2), showing a correlation factor of 0.97 with the surface area. Since IBU is a micromolecular drug,32 its loading capacity probably depends on the surface area instead of the pore size.33



