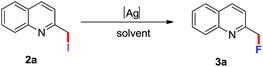Halogenations of substituted 2-alkylquinoline with iodine and halide exchange with AgF2†
Xiaobo Pang,
Likui Xiang,
Jianxin Ma,
Xiaodong Yang and
Rulong Yan*
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, Department of Chemistry, Lanzhou University, Lanzhou, China. E-mail: yanrl@lzu.edu.cn; Fax: +86-0931-8912596
First published on 21st November 2016
Abstract
Halogenations of substituted 2-alkylquinoline with iodine and halide exchange with AgF2 have been developed. These processes provide facile strategies to construct C–I/F bonds, and expand the methods of fluorination of 2-alkylquinolines. These transformations exhibit good tolerance of functional groups and generate the desired products in moderate to good yields.
Organic molecules containing halogenated functional groups are recognized as a crucial class of scaffolds, which are widely applied in pharmaceutical chemistry, agrochemistry and functional material science.1,2 In addition, organohalogen compounds are also used as synthetic precursors for organic synthesis.3 In view of these benefits, the development of carbon–halogen bonds formation has attracted substantial interest and made enormous progress during the past several years.4,5 Particularly, organic molecules containing fluorine atoms have emerged as star products in many fields due to the unique characteristics of carbon–fluorine bond,6 such as steric size effect, strong bond energy, change of lipophilicity, hydrogen bond acceptor, biological activity and high electronegativity. As consequence, continuous efforts have been devoted for the fluorination in the past several decades, as a rule, there are three general strategies for the construction of carbon–fluorine bond: nucleophilic,7 electrophilic,8 and radical fluorination.9 And more recently, success in this area can be attributed to the development of various efficient transition-metal catalysts, such as Pd,10 Cu,11 Ag,12 Au,13 which have been employed widely in the construction of C–F bond processes. Li,14 Sanford,15 Furuya,16 Hu,17 Ribas,18 Doyle,19 Buchwald,20 Nguyen,21 Gouverneur22 et al. have contributed greatly to the transition-metal-catalyzed C–F bond formation. Another important progress has also been investigated in the formation of C–F bonds based on the direct halide exchange. For instance, in 2012, Hartwig's group reported a new strategy of copper-mediated fluorination of aryl iodides23 (Scheme 1, entry A). Liu24 and Szabó25 respectively reported the fluorination of allylic halides by using Et3N·HF and (Ph3P)3CuF as the fluorine sources in the same year (Scheme 1, entry B). In contrast to recent conspicuous progress in formation of aromatic C(sp2)–F bond,26 the strategies of benzylic C(sp3)–F bond construction of 2-alkylquinolines are relatively limited.27 Therefore, the development of briefness methods to construction of C(sp3)–F bond of 2-alkylquinolines are very desirable and useful.
To our best knowledge, we found there was very few available literature concerning the methods of C(sp3)–I/F bonds construction of 2-alkylquinolines and their derivatives.28 Inspired by the previous works, herein, we had developed facile methods for C(sp3)–I/F bonds formation of substituted 2-alkylquinolines with iodine and halide exchanging by AgF2 (Scheme 1).
Firstly, 2-methylquinoline 1a was used as the model substrate to start our research. The expected product 2-(iodomethyl)quinoline 2a was isolated in 33% yield when the reaction was conducted with I2 (1.5 equiv.) and NaHCO3 (1.5 equiv.) in THF at 100 °C under N2 for 1.5 h (entry 1, Table 1). Encouraged by this initial result, we subsequently assessed Lewis bases as additives to improved the yield of this reaction (entry 2–3, Table 1), PPh3 promoted this reaction efficiently and the yield increased to 51%. The reaction temperature was also screened, 120 °C had been proven to be the efficient temperature in this process (entry 4–5, Table 1). Then several iodination reagents, such as tetrabutylammonium iodide (TBAI) and N-iodosuccinimide (NIS), were evaluated and only trace 2a were detected (entry 6–7, Table 1). Other bases were also examined, lower yields of 2a were obtained (entry 8–10, Table 1). Changing the solvent and the equivalent of I2 did not improve the yields (entry 11–13, Table 1). Consequently, the optimized reaction conditions should be proceeded with I2 (1.5 equiv.), NaHCO3 (1.5 equiv.) and PPh3 (10 mol%) in THF at 120 °C.
| Entry | Iodination reagent | Additive (10 mol%) | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.30 mmol), iodination reagent (1.5 equiv.), NaHCO3 (1.5 equiv.), PPh3 (10 mol%), THF (2 mL), 120 °C, under N2.b Isolated yields.c Reaction temperature was 100 °C.d Reaction temperature was 140 °C.e TBHP (1.5 equiv.).f The amount of I2 and NaHCO3 was increased to 2.0 equiv. | |||||
| 1 | I2 | — | NaHCO3 | THF | 33c |
| 2 | I2 | PPh3 | NaHCO3 | THF | 51c |
| 3 | I2 | 1,10-Phenanthroline | NaHCO3 | THF | n.r.c |
| 4 | I2 | PPh3 | NaHCO3 | THF | 77 |
| 5 | I2 | PPh3 | NaHCO3 | THF | 34d |
| 6 | TBAI/TBHP | PPh3 | NaHCO3 | THF | —e |
| 7 | NIS | PPh3 | NaHCO3 | THF | Trace |
| 8 | I2 | PPh3 | K2CO3 | THF | — |
| 9 | I2 | PPh3 | Na2CO3 | THF | — |
| 10 | I2 | PPh3 | NaOH | THF | 48 |
| 11 | I2 | PPh3 | NaHCO3 | DCE | 65 |
| 12 | I2 | PPh3 | NaHCO3 | Toluene | 51 |
| 13 | I2 | PPh3 | NaHCO3 | THF | 72f |
With the optimized reaction conditions in hand, the scope of substituted 2-alkylquinolines was examined and the results were summarized in Table 2. A wide series of substrates were tolerated in this transformation and gave desired products in moderate yields. Obviously, substituted 2-alkylquinolines with electron-donating groups, such as methyl and alkoxy groups, generated the iodination products in moderate yields. Nevertheless, the substrates with electron-withdrawing groups of fluorine, chlorine and bromine needed longer reaction time and failed to give ideal yields except these groups at C7 position (2j, 2l). 3-Methylbenzo[f]quinoline 1o was employed for this process under the standard conditions and product 2o was isolated in 68% yield. In addition, substrates 1p, 1q and 1r displayed better compatibility and gave desired products in moderate yields. Unfortunately, the benzylic C(sp3)–H of 1s and 1t could not be activated and give the unexpected products 2s and 2t. 2-Methylpyridine 1u and substrate 2v were also failed to give the desired product.
In view of the importance of fluorination, we expected that the halide exchange of 2a would be an ideal method for specific position C–F bond formation. To probe the viability of this approach, we started our research with the reaction of 2a and AgF in DMF at 120 °C. To our delight, the desired product 2-(fluoromethyl)quinoline 3a was obtained in 31% yield (entry 1, Table 3). The yield was increased to 54% when AgF2 was used as fluoride reagent (entry 2, Table 3). Further optimization of solvents demonstrated that toluene was the efficient solvent (entry 4–9, Table 3). After screening other parameters such as reaction temperature and time, the optimized reaction conditions should be performed in the presence of AgF2 in toluene at 80 °C for 16 h.
| Entry | Fluorination reagent | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 2a (0.30 mmol), AgF2 (1.2 equiv.), toluene (1.5 mL) at 80 °C under N2, 16 h.b Isolated yields.c Reaction temperature was 120 °C.d Reaction temperature was 40 °C. | |||
| 1c | AgF | DMF | 31 |
| 2 | AgF2 | DMF | 54 |
| 3d | AgF2 | DMF | 43 |
| 4 | AgF2 | DMF | 51 |
| 5 | AgF2 | CH3CN | 51 |
| 6 | AgF2 | DCE | 65 |
| 7 | AgF2 | THF | — |
| 8 | AgF2 | Toluene | 89 |
| 9 | AgF2 | DMSO | Trace |
Under the optimized reaction conditions, we then proceeded to investigate the scope of 2-(iodoalkyl 1,10-phenanthroline quinolines) (Table 4). To our delight, various substituted iodoalkylquinolines reacted with AgF2 well and generated the desired products in moderate to high yields. In this process, the presence of electron-donating and electron-withdrawing groups had very little influence on this transformation (3a–3n).
| a Reaction conditions: 2a (0.30 mmol), AgF2 (1.2 equiv.), toluene (1.5 mL) at 80 °C under N2, 16 h. |
|---|
 |
As shown in Table 4, substrate 2o was also tolerated in this process and the target compound 3o was obtained in excellent yield. When 2-(1-iodoethyl)quinoline 2p and 4-iodo-1,2,3,4-tetrahydroacridine 2r reacted with AgF2 under the optimized conditions, the desired products 3p and 3r were afforded in moderate yields. Additionally, substrate 2q failed to give target product 3q.
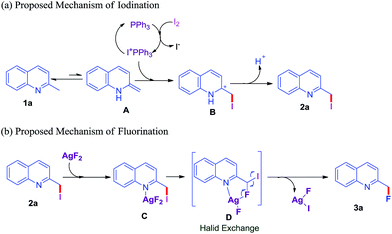
In order to gain further information about the reaction mechanism, several control experiments were set up. First, to determine whether 2-methylquinoline 1a rendered into 2-(iodomethyl)quinoline 2a through a sequence of radical pathway in this transformation, radical scavengers 2,2,6,6,-tetramethylpiperidyl-1-oxyl (TEMPO) and 2,6-di-tert-butyl-4-methylphenol (BHT) were respectively performed with 2-methylquinoine 1a under the standard conditions (Scheme 2, entry A), the corresponding product 2a were separately isolated in 71% and 53% yields, which ruled out that the iodination process might proceed through a radical pathway. Then, further experimental study was carried out to confirm the mechanism for fluorination of 2a. TEMPO was added into the reaction system of (iodomethyl)quinoline 2a and AgF2 in toluene at 80 °C under N2, and gave the corresponding product 3a in 85% yield (Scheme 2B), which indicated that the (iodomethyl)quinoline 2a transformed to the target product 3a without a radical procedure. Based on the above experiment results, a possible reaction mechanism of iodination was proposed and summarized in Scheme 2. Firstly, substrate 1a equilibrated to generate intermediate A through tautomerization under the optimized conditions. Then, iodine underwent heterolytic cleavage in presence of PPh3 to form cation Ph3PI+. Then Ph3PI+ attacked A to form the intermediate B through electrophilic addition. Finally, the product 2a was achieved by the procedures of proton elimination. Meanwhile, we also proposed the plausible mechanism of fluorination between 2a and AgF2 (Scheme 2). Incipiently, substrate 2a would involve coordination with AgF2 to produce the pyridine–AgF2 complex C.29 Then, the complex C went through a consecutive state of intermediate D, afforded the corresponding product 3a via halide exchange, and Ag(II)-intermediate was also generated at the same time (Scheme 3).
In summary, we have developed simple methods of iodination/fluorination of substituted 2-alkylquinolines. The substrates with different functional groups, such as methyl, methoxyl, bromo, chloro and fluoro, were performed smoothly in the process of iodination/fluorination and formed target products in good yields under mild conditions.
Acknowledgements
This work was supported by National Natural Science Foundation of China (21672086, 21202067), Gansu Province Science Foundation for Youths (1606RJYA260) and the Fundamental Research Funds for the Central Universities (lzujbky-2016-39).Notes and references
- (a) K. Muller, C. Faeh and F. Diederich, Fluorine in pharmaceuticals: looking beyond intuition, Science, 2007, 317, 1881 CrossRef PubMed; (b) S. Purser, P. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (c) K. L. Kirk, Org. Process Res. Dev., 2008, 12, 305 CrossRef CAS.
- (a) V. V. Kouznetsov, L. Y. Mendez and C. M. Gomez, Curr. Org. Chem., 2005, 9, 141 CrossRef CAS; (b) J. I. Kim, I. S. Shin, H. Kim and J. K. Lee, J. Am. Chem. Soc., 2005, 127, 1614 CrossRef CAS PubMed; (c) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166 RSC; (d) V. R. Solomon and H. Lee, Curr. Med. Chem., 2011, 18, 1488 CrossRef CAS PubMed.
- (a) P. Knochel, W. Dohle, N. Gommermann, F. F. Kneisel, T. Korn, I. Sapountzis and V. A. Vu, Angew. Chem., Int. Ed., 2003, 42, 4302 CrossRef CAS PubMed; (b) T. D. Sheppard, Org. Biomol. Chem., 2009, 7, 1043 RSC; (c) N. Kambe, T. Iwasaki and J. Terao, Chem. Soc. Rev., 2011, 40, 4937 RSC.
- (a) T. S. Mei, D. H. Wang and J. Q. Yu, Org. Lett., 2010, 12, 3140 CrossRef CAS PubMed; (b) W. A. Nack, G. He, S. Y. Zhang, C. X. Lu and G. Chen, Org. Lett., 2013, 15, 3440 CrossRef CAS PubMed; (c) R. Möckel and G. Hilt, Org. Lett., 2015, 17, 1644 CrossRef PubMed.
- (a) C. Xue, X. P. Jiang, C. L. Fu and S. M. Ma, Chem. Commun., 2013, 49, 5651 RSC; (b) T. Fujita, K. Sugiyama, S. Sanada, T. Ichitsuka and J. Ichikawa, Org. Lett., 2016, 18, 248 CrossRef CAS PubMed.
- (a) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis Reactivity, Application, Wiley-VCH, Weinheim, 2004 Search PubMed; (b) T. Hiyama, K. Kanie, T. Kusumoto, Y. Morizawa and M. Shimizu, Organofluorine Compounds: Chemistry and Applications, Springer, Berlin, 2000 Search PubMed; (c) D. O'Hagan and H. S. Rzepa, Chem. Commun., 1997, 645 RSC; (d) B. E. Smart, J. Fluorine Chem., 2001, 109, 3 CrossRef CAS; (e) F. M. D. Ismail, J. Fluorine Chem., 2002, 118, 27 CrossRef CAS; (f) P. T. Nyffeler, S. G. Duron, M. D. Burkart, S. P. Vincent and C. H. Wong, Angew. Chem., Int. Ed., 2005, 44, 192 CrossRef CAS PubMed; (g) M. Shimizu and T. Hiyama, Angew. Chem., Int. Ed., 2005, 44, 214 CrossRef CAS PubMed; (h) V. D. Romanenko and V. P. Kukhar, Chem. Rev., 2006, 106, 3868 CrossRef CAS PubMed; (i) D. O'Hagan, Chem. Soc. Rev., 2008, 37, 308 RSC; (j) W. K. Hagmann, J. Med. Chem., 2008, 51, 43599 CrossRef PubMed.
- (a) H. J. Frohn and M. Giesen, J. Fluorine Chem., 1998, 89, 59 CrossRef CAS; (b) H. Sun and S. G. DiMagno, J. Am. Chem. Soc., 2005, 127, 2050 CrossRef CAS PubMed; (c) H. Sun and S. G. DiMagno, Chem. Commun., 2007, 528 RSC; (d) D. W. Kim, H. J. Jeong, S. T. Lim and M. H. Sohn, Angew. Chem., Int. Ed., 2008, 47, 8404 CrossRef CAS PubMed; (e) S. Hara, M. Monoi, R. Umemura and C. Fuse, Tetrahedron, 2012, 68, 10145 CrossRef CAS; (f) T. Shishimi and S. Hara, J. Fluorine Chem., 2014, 168, 55 CrossRef CAS; (g) O. E. Okoromoba, J. Han, G. B. Hammond and B. Xu, J. Am. Chem. Soc., 2014, 136, 14381 CrossRef CAS PubMed; (h) W. J. Middleton, J. Org. Chem., 1975, 40, 574 CrossRef CAS; (i) R. P. Singh and J. M. Shreeve, Synthesis, 2002, 2561 CAS; (j) T. Umemoto, R. P. Singh, Y. Xu and N. Saito, J. Am. Chem. Soc., 2010, 132, 18199 CrossRef CAS PubMed; (k) T. Umemoto and R. P. Singh, J. Fluorine Chem., 2012, 140, 17 CrossRef CAS; (l) F. Beaulieu, L. P. Beauregard, G. Courchesne, M. Couturier and F. Laflamme, Org. Lett., 2009, 11, 5050 CrossRef CAS PubMed; (m) A. L'Heureux, F. Beaulieu, C. Bennet, D. R. Bill, S. Clayton, F. Laflamme, M. Mirmehrabi, S. Tadayon, D. Tovell and M. Couturier, J. Org. Chem., 2010, 75, 3401 CrossRef PubMed; (n) O. Mahe, A. L'Heureux, M. Couturier, C. Bennett, S. Clayton, D. Tovell, F. Beaulieu and J. F. Paquin, J. Fluorine Chem., 2013, 153, 57 CrossRef CAS; (o) P. Tang, W. Wang and T. Ritter, J. Am. Chem. Soc., 2011, 133, 11482 CrossRef CAS PubMed; (p) F. Sladojevich, S. I. Arlow, P. Tang and T. Ritter, J. Am. Chem. Soc., 2013, 135, 2470 CrossRef CAS PubMed.
- (a) P. T. Nyffeler, S. G. Duron, M. D. Burkart, S. P. Vincent and C. H. Wong, Angew. Chem., Int. Ed., 2004, 44, 192 CrossRef PubMed; (b) A. S. Kiselyov, Chem. Soc. Rev., 2005, 34, 1031 RSC; (c) J. Baudoux and D. Cahard, Org. React., 2007, 69, 1 Search PubMed; (d) R. P. Singh and J. M. Shreeve, Acc. Chem. Res., 2004, 37, 31 CrossRef CAS PubMed; (e) H. Yasui, T. Yamamoto, T. Ishimaru, T. Fukuzumi, E. Tokunaga, K. Akikazu, M. Shiro and N. Shibata, J. Fluorine Chem., 2011, 132, 222 CrossRef CAS; (f) G. C. Geary, E. G. Hope, K. Singh and A. M. Stuart, Chem. Commun., 2013, 49, 9263 RSC; (g) J. R. Wolstenhulme, J. Rosenqvist, O. Lazano, J. Ilupeju, N. Wurz, K. M. Engle, G. W. Pidgeon, P. R. Moore, G. Sandford and V. Gouverneur, Angew. Chem., Int. Ed., 2013, 52, 9796 CrossRef CAS PubMed; (h) C. L. Zhu, M. Maeno, F. G. Zhang, T. Shigehiro, T. Kagawa, K. Kawada, N. Shibata, J. A. Ma and D. Cahard, Eur. J. Org. Chem., 2013, 6501 CrossRef CAS; (i) F. Wang, J. Li, Q. Hu, X. Yang, X. Y. Wu and H. He, Eur. J. Org. Chem., 2014, 3067 Search PubMed.
- (a) S. Yamada, A. Gavryushin and P. Knochel, Angew. Chem., Int. Ed., 2010, 49, 2215 CrossRef CAS PubMed; (b) M. Dobele, S. Vanderheiden, N. Jung and S. Brase, Angew. Chem., Int. Ed., 2010, 49, 5986 Search PubMed; (c) M. Rueda-Becerril, C. Chatalova-Sazepin, J. C. T. Leung, T. Okbinoglu, P. Kennepohl, J. F. Paquin and G. M. Sammis, J. Am. Chem. Soc., 2012, 134, 4026 CrossRef CAS PubMed; (d) J. C. T. Leung, C. Chatalov-Sazepin, J. G. West, M. Rueda-Becerril, J. F. Paquin and G. M. Sammis, Angew. Chem., Int. Ed., 2012, 51, 10804 CrossRef CAS PubMed.
- (a) K. M. Engle, T. S. Mei, X. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2011, 50, 1478 CrossRef CAS PubMed; (b) T. Furuya, H. M. Kaiser and T. Ritter, Angew. Chem., Int. Ed., 2008, 47, 5993 CrossRef CAS PubMed; (c) T. Furuya and T. Ritter, J. Am. Chem. Soc., 2008, 130, 10060 CrossRef CAS PubMed; (d) N. D. Ball and M. S. Sanford, J. Am. Chem. Soc., 2009, 131, 3796 CrossRef CAS PubMed; (e) X. Wang, T.-S. Mei and J. Q. Yu, J. Am. Chem. Soc., 2009, 131, 7520 CrossRef CAS PubMed; (f) C. Lu, S. Y. Zhang, G. He, W. A. Nack and G. Chen, Tetrahedron, 2014, 70, 4197 CrossRef CAS; (g) K. L. S. Chan, M. Wasa, X. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2011, 50, 9081 CrossRef CAS PubMed; (h) S. J. Lou, D. Q. Xu, A. B. Xia, Y. F. Wang, Y. K. Liu, X. H. Du and Z. Y. Xu, Chem. Commun., 2013, 49, 6218 RSC; (i) Q. Ding, C. Ye, S. Pu and B. Cao, Tetrahedron, 2014, 70, 409 CrossRef CAS.
- (a) M. A. Subramanian and L. E. Manzer, Science, 2002, 297, 1665 CrossRef CAS PubMed; (b) T. Truong, K. Klimovica and O. Daugulis, J. Am. Chem. Soc., 2013, 135, 9342 CrossRef CAS PubMed; (c) N. Ichiishi, A. J. Canty, B. F. Yates and M. S. Sanford, Org. Lett., 2013, 15, 5134 CrossRef CAS PubMed; (d) J. Zhang, H. Wang, S. Ren, W. Zhang and Y. Liu, Org. Lett., 2015, 17, 2920 CrossRef CAS PubMed.
- J. Qian, Y. Liu, J. Zhu, B. Jiang and Z. Xu, Org. Lett., 2011, 13, 4220 CrossRef CAS PubMed.
- (a) J. A. Akana, K. X. Bhattacharyya, P. Müller and J. P. Sadighi, J. Am. Chem. Soc., 2007, 129, 7736 CrossRef CAS PubMed; (b) B. C. Gorske, C. T. Mbofana and S. J. Miller, Org. Lett., 2009, 11, 4318 CrossRef CAS PubMed; (c) T. De Haro and C. Nevado, Chem. Commun., 2011, 47, 248 RSC.
- (a) F. Yin, Z. T. Wang, Z. D. Li and C. Z. Li, J. Am. Chem. Soc., 2012, 134, 10401 CrossRef CAS PubMed; (b) C. W. Zhang, Z. D. Li, L. Zhu, Z. T. Wang and C. Z. Li, J. Am. Chem. Soc., 2013, 135, 14082 CrossRef CAS PubMed; (c) Z. D. Li, L. Y. Song and C. Z. Li, J. Am. Chem. Soc., 2013, 135, 4640 CrossRef CAS PubMed.
- Y. Ye, S. D. Schimler, P. S. Hanley and M. S. Sanford, J. Am. Chem. Soc., 2013, 135, 16292 CrossRef CAS PubMed.
- (a) T. Furuya and T. Ritter, J. Am. Chem. Soc., 2008, 130, 12834 CrossRef CAS; (b) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef CAS PubMed; (c) A. R. Mazzotti, M. G. Campbell, P. Tang, J. M. Murphy and T. Ritter, J. Am. Chem. Soc., 2013, 135, 14012 CrossRef CAS PubMed.
- B. Gao, Y. C. Zhao and J. B. Hu, Angew. Chem., Int. Ed., 2015, 54, 638 CAS.
- A. Casitas, M. Canta, M. Sola, M. Costas and X. Ribas, J. Am. Chem. Soc., 2011, 133, 19386 CrossRef CAS PubMed.
- (a) M. H. Katcher and A. G. Doyle, J. Am. Chem. Soc., 2010, 132, 17402 CrossRef CAS PubMed; (b) M. H. Katcher, A. Sha and A. G. Doyle, J. Am. Chem. Soc., 2011, 133, 15902 CrossRef CAS PubMed; (c) M. G. Braun and A. G. Doyle, J. Am. Chem. Soc., 2013, 135, 12990 CrossRef CAS PubMed.
- (a) P. J. Milner, T. Kinzel, Y. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2014, 136, 15757 CrossRef CAS PubMed; (b) A. C. Sather, H. G. Lee, V. Y. De La Rosa, Y. Yang, P. Müller and S. L. Buchwald, J. Am. Chem. Soc., 2015, 137, 13433 CrossRef CAS PubMed; (c) T. Noel, T. J. Maimone and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 8900 CrossRef CAS PubMed.
- (a) J. J. Topczewski, T. J. Tewson and H. M. Nguyen, J. Am. Chem. Soc., 2011, 133, 19318 CrossRef CAS PubMed; (b) Q. Zhang, D. P. Stockdale, J. C. Mixdorf, J. J. Topczewski and H. M. Nguyen, J. Am. Chem. Soc., 2015, 137, 11912 CrossRef CAS PubMed.
- (a) S. Thibaudeau and V. Gouverneur, Org. Lett., 2003, 5, 4891 CrossRef CAS PubMed; (b) S. Mizuta, I. S. R. Stenhagen, M. O'Duill, J. Wolstenhulme, A. K. Kirjavainen, S. J. Forsback, M. Tredwell, G. Sandford, P. R. Moore, M. Huiban, S. K. Luthra, J. Passchier, O. Solin and V. Gouverneur, Org. Lett., 2013, 15, 2648 CrossRef CAS PubMed.
- P. S. Fier and J. F. Hartwig, J. Am. Chem. Soc., 2012, 134, 10795 CrossRef CAS PubMed.
- Z. Zhang, F. Wang, X. Mu, P. Chen and G. Liu, Angew. Chem., Int. Ed., 2013, 52, 7549 CrossRef CAS PubMed.
- J. M. Larsson, S. R. Pathipati and K. J. Szabó, J. Org. Chem., 2013, 78, 7330 CrossRef CAS PubMed.
- (a) D. V. Yandulov and N. T. Tran, J. Am. Chem. Soc., 2007, 129, 1342 CrossRef CAS PubMed; (b) Y. Ye, S. D. Schimler, P. S. Hanley and M. S. Sanford, J. Am. Chem. Soc., 2013, 135, 16292 CrossRef CAS PubMed; (c) S. J. Lou, D. Q. Xu, A. B. Xia, Y. F. Wang, Y. K. Liu, X. H. Du and Z. Y. Xu, Chem. Commun., 2013, 49, 6218 RSC; (d) Q. Ding, C. Ye, S. Pu and B. Cao, Tetrahedron, 2014, 70, 409 CrossRef CAS.
- P. A. Champagne, J. Desroches, J. D. Hamel, M. Vandamme and J. F. Paquin, Chem. Rev., 2015, 115, 9073 CrossRef CAS PubMed.
- (a) M. C. Kimber, I. B. Mahadevan, S. F. Lincoln, A. D. Ward and E. R. T. Tiekink, J. Org. Chem., 2000, 65, 8204 CrossRef CAS PubMed; (b) P. Warner, A. J. Barker, A. L. Jackman, K. D. Burrows, N. Roberts, J. A. M. Bishop, B. M. OConnor and L. R. Hughest, J. Med. Chem., 1992, 35, 2761 CrossRef CAS PubMed; (c) A. Mobinikhaledi and N. Foroughifar, Phosphorus, Sulfur Silicon Relat. Elem., 2006, 181, 405 CrossRef CAS.
- P. S. Fier and J. F. Hartwig, Science, 2013, 342, 956 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data. See DOI: 10.1039/c6ra17410h |
| This journal is © The Royal Society of Chemistry 2016 |