Open Access Article
Open Access ArticleAre specific buffer effects the new frontier of Hofmeister phenomena? Insights from lysozyme adsorption on ordered mesoporous silica†‡
Francesca Cugiaa,
Silvia Seddaa,
Federica Pitzalisa,
Drew F. Parsonsb,
Maura Monduzzi*a and
Andrea Salis *a
*a
aDepartment of Chemical and Geological Sciences, University of Cagliari-CSGI and CNBS, Cittadella Universitaria, S.S. 554 Bivio Sestu, 09042-Monserrato, CA, Italy. E-mail: asalis@unica.it; monduzzi@unica.it
bSchool of Engineering and Information Technology, Murdoch University, 90 South St, Murdoch, WA 6150, Australia
First published on 28th September 2016
Abstract
Lysozyme adsorption on mesoporous silica at pH 7.15 is buffer specific. The synergistic action of buffers and salts induces relevant effects on the charged interfaces, and thus on lysozyme loading. These findings, rising doubts on the validity of the Henderson–Hasselbalch equation, suggest the occurrence of Hofmeister phenomena also for buffers.
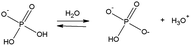
Hofmeister (ion specific) effects are phenomena related to the chemical nature of electrolytes. Although they are ubiquitous in all chemical, colloidal, and biological systems,1–5 they cannot be quantified in terms of the conventional physico-chemical theories (i.e. Debye–Hückel, DLVO, etc.). These are limit theories, based on electrostatics, and valid at infinite dilution only. The gap between theories and Hofmeister related experiments is usually very large since the ion specific effects are generally observed at high concentrations in the presence of strong electrolytes 0.3–3 M.6 This is very far from the validity's domain of both limit laws, and their extensions (valid up to 10−3 to 10−2 M).3 Nevertheless, it has been demonstrated that high concentrations of strong electrolytes are not strictly necessary to observe ion dependent phenomena. Several experiments showed that ion specificity occurs at physiological salt concentrations (0.1–0.15 M), and even below.7,8 Very recently, the occurrence of ion specificity at low salt concentrations (Hofmeister charging) has found a theoretical basis.9 The finding that Hofmeister effects occur at low salt concentrations poses new questions. Can the weak electrolytes used for buffers give Hofmeister effects? According to the Henderson–Hasselbalch equation,10 they should not. In biochemistry, it is necessary to use a buffer to fix the pH of the experiment. This procedure has been used in many ‘Hofmeister related’ studies, in particular on protein aggregation, electrophoretic mobility and enzyme activity.11–15 Typical buffer concentrations are in the range 10–100 mM. The implicit assumption is that, due to their low concentration, the buffer ions should not display any specific effect. This is not so. Indeed we recently observed that, even at the same nominal pH, lysozyme electrophoretic mobility was buffer dependent.16 The conventional wisdom says that protein charges – and hence electrophoretic mobilities – depend on pH only, regardless of the buffer used to fix it. As shown below, here we confirmed and extended that work.16 It should be acknowledged that the original idea to investigate specific buffer effects is due to B. W. Ninham. Following his pioneering work on restriction enzymes,17 we then observed specific buffer effects on pH measurements,18 and lipase activities.19 Moreover, by looking at the literature more in detail,20 we found that similar specific buffer effects were discerned for DNA mobility,21 protein stability,22–24 antibody aggregation,25,26 swelling kinetics of polyelectrolyte gels27 etc. Hence, buffers, besides setting pH, may specifically interact with surfaces to modulate their effective charges.20 This is not considered in the Henderson–Hasselbalch model for buffer action.28
Ordered mesoporous silica (OMS) materials have shown a very high propensity towards the adsorption of proteins and enzymes.29–31 The most common OMS materials (i.e. MCM-41, SBA-15), besides a very high surface area, have pore sizes in the range 2–10 nm. These features allow for the penetration of proteins inside the inner architecture of the OMS particles.32,33 When an enzyme is adsorbed on OMS an immobilised biocatalyst is obtained.34 In nanomedicine applications, OMS can be used to adsorb antibodies or other proteins which either act as targeting agents (molecular recognition), or as therapeutics which require a sustained release.35 The potentiality of OMS–proteins composites has promoted a growing interest in different applied fields.35 In these systems protein/silica interfacial interactions play a fundamental role. It is widely acknowledged that the pH and the ionic strength of the adsorbing solution affect the interactions.36–38 Less investigated, but not really surprising, is that ions affect specifically protein adsorption on silica.39 Generally ‘ion-specific’ works are carried out by fixing pH by means of a buffer and then varying the salt type and/or concentration.11–13 Here, our purpose is to demonstrate that, besides strong electrolytes, also weak electrolytes, used to fix pH, play a specific role. This has not yet been investigated systematically. The system chosen to this purpose was the physical immobilisation of lysozyme (LYZ) on SBA-15 and amino functionalised SBA-15 (SBA-NH2), which we studied in the presence of different buffers (pH 7.15) and different 0.1 M salts. The performed experiments are described in the ESI file‡ and schematised in Scheme 1.
Briefly, LYZ adsorption on SBA-15 (or SBA-NH2) mesoporous silica was carried out at 298 K and pH 7.15 in the presence of different buffers (Tris, BES, phosphate, and citrate), and quantified through spectrophotometry at λ = 280 nm. Buffers dissociation equilibria and pKa values are listed in Table 1. Phosphate and citrate are typical buffers occurring in living systems. Tris and BES are commonly used in biochemistry labs. The pKas values listed in Table 1, confirm that all buffers would have a good ‘buffering action’ at a physiological pH = 7–7.4. LYZ adsorption was carried out also in the presence of different 100 mM sodium salts, namely: NaCl, NaNO3 and NaSCN (and for some experiments also NaBr and NaI). Firstly, the SBA-15 and SBA-NH2 adsorbent materials were synthesised and characterised according to the procedures reported in the ESI file.‡ Textural characterisation of SBA-15 (Fig. S1 in ESI‡) provided a surface area (BET) of 777 m2 g−1, and a maximum of the pore size distribution (BJH) at 6.4 nm (Table S1 in ESI‡). The functionalisation with aminopropyl group to obtain SBA-NH2 resulted in a decrease of both surface area to 398 m2 g−1, and pore size to 5.9 nm. The structural characterisation, carried out through SAXS, showed that both SBA-15 and SBA-NH2 have an ordered hexagonal structure with a lattice parameter of 11.7 nm and 11.6 nm, respectively (Fig. S1C and D, ESI‡). The functionalisation with the aminopropyl group produced a change of the sign of the silica surface charge, as demonstrated through potentiometric titrations. The curves of surface charge density (σ) as a function of pH (Fig. S1F, ESI‡) (obtained in the absence of buffers or other electrolytes) showed that, at pH 7.15, σ of SBA-15 is negative (−0.04 C m−2), while that of SBA-NH2 is positive (+0.23 C m−2). This means that, in the absence of any added electrolyte, the two materials have opposite surface charges. Therefore we can expect that the adsorption of LYZ (isoelectric point: pI = 11) is much more favoured on SBA-15 than on SBA-NH2.
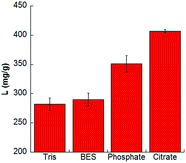
This is demonstrated in Fig. 1 where LYZ adsorption is reported for the two silica matrices in the presence of different 10 mM buffers, at the same pH 7.15. In the case of SBA-15 (Fig. 1A) LYZ loading is always higher than 250 mg g−1, and increases along the series Tris < BES < phosphate < citrate. Interestingly, the use of citrate instead of Tris buffer, resulted in a loading increase of 44%. A very low loading (in the range 0–10 mg g−1) was instead obtained for LYZ adsorption on SBA-NH2. This was expected on the basis of electrostatic considerations. For comparison, we also measured LYZ loading by adjusting pH at about 7.15 with addition of strong acid/base. We obtained a loading of 159 ± 58 mg g−1 for SBA-15 and about zero for SBA-NH2. We assign the high uncertainties to the difficulty to control pH without using a buffer. From the results in Fig. 1 we can argue that the chemical nature of both silica surfaces and buffers is relevant in addressing the observed phenomena. Recent experiments suggested that buffer ions adsorb specifically at LYZ surface, thus modifying its effective electric charge, thus affecting its electrophoretic mobility.16 Zeta potential (ζ) measurements shown in Fig. 2 confirm those previous results. Indeed, at pH 7.15, LYZ carries a positive net charge thus a positive ζ value would be expected, independently of the buffer used to fix pH. But Fig. 2A shows that the ζ values depend significantly on the buffer, and decrease along the series Tris > BES > phosphate > citrate. The effect of buffers on ζ measurements was here investigated also for the silica matrices. In the case of SBA-15 (Fig. 2B) ζ values are always negative and do not depend on the different type of buffer significantly. On the contrary, in the case of SBA-NH2 (Fig. 2C), ζ values display a clear specific buffer dependence exactly as observed for LYZ (Tris > BES > phosphate > citrate). Remarkably, confirming what previously observed for LYZ,16 citrate gives a reversal of zeta potential from positive to negative values also for SBA-NH2. These results demonstrate that the specific effect of buffers is important for LYZ and SBA-NH2, but not for SBA-15. Hence, the effect of the buffer (at pH 7.15) can be related to the presence of the positively charged amino groups at the SBA-NH2 surface, and amino, imidazole and guanidino groups at LYZ surface.
 | ||
| Fig. 2 Specific buffer effects on zeta potential of (A) LYZ (B) SBA-15 (C) SBA-NH2 (T = 298 K; pH = 7.15; buffer concentration 10 mM). | ||
Differently, the negatively charged silanol (SiO−) groups of SBA-15 are negligibly affected by the different buffers. It is evident that buffers' anions, particularly citrate buffer (constituted by the divalent hydrogen citrate and the trivalent citrate anions, see Table 1) displays a very high affinity and selectivity towards binding at the positively charged interfaces. Hence, a combination of electrostatic, and van der Waals interactions could be suggested to justify the observed trends.36 In fact, a more detailed approach has recently been proposed.9,41 The effects of increasing the buffers' concentration (from 10 mM to 100 mM) on LYZ loading on SBA-15 was also studied, as shown in Fig. S2 (ESI‡). The increase of the buffers' concentration also affected the absolute value of zeta potentials of SBA-15 (see Fig. S3 ESI‡). However, the increase of buffer concentration did not provide any significant advantage to loading, therefore we used a 10 mM buffer concentration to evaluate the effect of adding strong electrolytes.
Different 100 mM sodium salts were added during the LYZ loading experiments on SBA-15 and SBA-NH2. The investigated anions were Cl− and SCN−, that are located respectively in the middle and at the ‘chaotropic end’ of the Hofmeister series, and NO3− which is in between these two. Fig. 3 shows the effect of the salt addition on ζ-potentials of LYZ in different buffer solutions. The salt addition produces negative ζ values on LYZ in the presence of all buffers except BES, probably due to the zwitterionic nature of this buffer. Fig. 4A and B show the effect of salt addition on ζ-potential of the two silica matrices in the presence of Tris (cationic) and citrate (anionic) buffers. These buffers were selected since, in the absence of added salt, they gave the lowest and the highest LYZ loading on SBA-15, respectively (Fig. 1). In the case of SBA-15, the addition of salt just decreases the absolute value of ζ-potentials as a result of cations' binding to the negatively charged silica surface.
For SBA-NH2 besides the effect of NaCl, NaNO3, and NaSCN, we also investigated the effect of NaBr and NaI, as shown in Fig. 4B. Also in this case the addition of 100 mM sodium salts decreases ζ-potentials, but a ‘bell shaped’ Hofmeister series is observed (when anions are ordered by the conventional series) in the presence of both buffers. Interestingly, SBA-NH2 sample is more sensitive than SBA-15 to both buffer and salt type as shown by the corresponding ζ-potentials. Definitely, the addition of salts reduces significantly the surface charge in all cases, but does not cancel the effect of buffers in the cases of the two positively charged surfaces of LYZ and SBA-NH2.
Finally, let us focus on LYZ loading in the presence of buffers and salts. Fig. 5 shows the results for the two silica matrices in Tris and citrate buffers: for SBA-NH2 the effects of NaBr and NaI are also enclosed. In the case of SBA-15 (Fig. 5A) in Tris buffer a LYZ loading increase along the series ‘No Salt’ < NaCl < NaBr ≪ NaSCN was observed. In citrate buffer, instead, ions specificity was lost. Evidently, the background buffer, used to set the pH, triggers a further dependence on the anion type of the added strong electrolytes. In the case of SBA-NH2, LYZ loading data, in the presence of NaCl, NaBr, and NaNO3, are very low (<20 mg g−1). Strikingly, the addition of either NaI or NaSCN induced a huge increase of loading. This high loading is achieved for both buffers (and also for BES and phosphate buffers: see Fig. S4 in ESI‡). Fig. 5 shows another noteworthy result: in the presence of 100 mM NaSCN (or NaI), LYZ loading obtained for SBA-15 (Tris buffer) and SBA-NH2 (all buffers) is very similar. In a recent interesting work Meissner et al. proposed a model for the calculation of the maximum uptake capacity of SBA-15.42 A rough comparison between the adsorption data of the present and that work would lead to the conclusion that the very high loading obtained here for SBA-15 and SBA-NH2 in the presence of 0.1 M NaSCN (or NaI) exceed the maximum loading capacity. In fact, although the Meissner model is highly reasonable, this is based on geometric considerations of pore size and pore volume of the sorbent material as well as LYZ size. There might be several explanations for that apparent inconsistency. In particular, we argue that the different method used to calculate the pore size might be the main cause.43 Indeed we used the BJH method (ESI‡), whereas the KJS method was used by Meissner et al.42 It has been shown that different methods can result in different pore sizes.44 Moreover, we cannot exclude that, in addition to adsorption, an unwanted LYZ co-precipitation phenomenon may occur. A systematic investigation of the reasons of the high loading observed in the presence of 0.1 M NaI and NaSCN is, although of extreme interest, beyond the scopes of this work which was mainly devoted to investigate the specific effect of buffers.
 | ||
| Fig. 5 Effect of buffers (10 mM) and added salts (100 mM) on LYZ loading at 298 K and pH 7.15 on (A) SBA-15; (B) SBA15-NH2. | ||
The high LYZ loading observed for SBA-NH2 found in the presence of the most polarisable anions I− and SCN−, might be due to their ability to counteract, and exceed both the electrostatic repulsion between the positively charged LYZ and SBA-NH2 surfaces. Definitely, the effect of NaSCN (and NaI) is so strong to overcome all other types of interactions or buffer specific effects involved in LYZ loading. This striking result cannot be justified in terms of electrostatic interactions only, since salt addition reduces significantly all surface charges, as demonstrated by ζ-potential data shown in Fig. 3 and 4. Indeed, the LYZ loading around 600–700 mg g−1 obtained for both positively and negatively charged silica matrices, is likely promoted by non-electrostatic interactions mainly. A recent work investigating the effect of ionic strength on the adsorption and release of lysozyme from SBA-15 at pH 7 (10 mM phosphate buffer),38 showed that a high ionic strength (1 M NaCl) favours the LYZ adsorption but not its release. Here we fixed pH (7.15) and ionic strength (100 mM) but varied the nature of the buffer used to set pH, the salt used to set ionic strength, and the sorbent surface. The fact that buffers and salt ions give rise to different loadings suggests that they interact specifically with LYZ and silica surface sites, thus affecting their actual dissociation constants and effective surface charges.9,41,45 The main outcome is that protein loading on OMS is the result of the complicated interplay among the specific effects of the buffers, the salts, and the charged interfaces. Sometimes the dominant effect is due to the buffer, sometimes to the salt, sometimes both matter. In this work the buffer effect is more important for LYZ loading on SBA-15 (Fig. 1), whereas the specific anion effect is strong also for SBA-NH2 sorbent (Fig. 5B).
Finally, we remark that the consequences of this work go beyond the specific case described here. The Henderson–Hasselbalch equation, known to be one of the pillars of chemistry, considers buffers action only in terms of the pKa of the weak electrolyte. But in the presence of charged interfaces the buffer species tend to adsorb thus modifying the effective surface charges and the physico-chemical behaviour of the system. Here, a competition between buffer and salt ions for adsorption on LYZ and OMS surfaces can clearly be inferred. Hence, specific buffer effects would require to be included in the plethora of phenomena that we classify as ‘Hofmeister effects’.
Acknowledgements
Fondazione Banco di Sardegna (PRID 2015) is acknowledged for financial support. F. P. Thanks Agenzia delle Dogane e dei Monopoli for financing her PhD scholarship.References
- P. Lo Nostro and B. W. Ninham, Chem. Rev., 2012, 112, 2286–2322 CrossRef CAS PubMed.
- F. Tadini-Buoninsegni, M. R. Moncelli, N. Peruzzi, B. W. Ninham, L. Dei and P. Lo Nostro, Sci. Rep., 2015, 5, 14282 CrossRef CAS PubMed.
- A. Salis and B. W. Ninham, Chem. Soc. Rev., 2014, 43, 7358–7377 RSC.
- C. Carucci, P. Haltenort, M. Salazar, A. Salis and E. Magner, ChemElectroChem, 2015, 2, 659–663 CrossRef CAS.
- B. W. Ninham and P. Lo Nostro, Molecular Forces and Self Assembly In Colloid, Nano Sciences and Biology, Cambridge University Press, Cambridge, 2010 Search PubMed.
- A. A. Green, J. Biol. Chem., 1932, 95, 47–66 Search PubMed.
- L. Medda, C. Carucci, D. F. Parsons, B. W. Ninham, M. Monduzzi and A. Salis, Langmuir, 2013, 29, 15350–15358 CrossRef CAS PubMed.
- L. Medda, M. Monduzzi and A. Salis, Chem. Commun., 2015, 51, 6663–6666 RSC.
- D. F. Parsons and A. Salis, Curr. Opin. Colloid Interface Sci., 2016, 23, 41–49 CrossRef CAS.
- R. de Levie, J. Chem. Educ., 2003, 80, 146 CrossRef CAS.
- Y. Zhang and P. S. Cremer, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15249–15253 CrossRef CAS PubMed.
- A. Salis, F. Cugia, D. F. Parsons, B. W. Ninham and M. Monduzzi, Phys. Chem. Chem. Phys., 2012, 14, 4343–4346 RSC.
- L. Medda, A. Salis and E. Magner, Phys. Chem. Chem. Phys., 2012, 14, 2875–2883 RSC.
- P. Lo Nostro, N. Peruzzi, M. Severi, B. W. Ninham and P. Baglioni, J. Am. Chem. Soc., 2010, 132, 6571–6577 CrossRef CAS PubMed.
- Y. R. Gokarn, R. M. Fesinmeyer, A. Saluja, V. Razinkov, S. F. Chase, T. M. Laue and D. N. Brems, Protein Sci., 2011, 20, 580–587 CrossRef CAS PubMed.
- F. Cugia, M. Monduzzi, B. W. Ninham and A. Salis, RSC Adv., 2013, 3, 5882–5888 RSC.
- H. Kim, E. Tuite, B. Norden and B. W. Ninham, Eur. Phys. J. E, 2001, 4, 411–417 CrossRef CAS.
- A. Salis, M. C. Pinna, D. Bilanicová, M. Monduzzi, P. Lo Nostro and B. W. Ninham, J. Phys. Chem. B, 2006, 110, 2949–2956 CrossRef CAS PubMed.
- A. Salis, D. Bilanicová, B. W. Ninham and M. Monduzzi, J. Phys. Chem. B, 2007, 111, 1149–1156 CrossRef CAS PubMed.
- A. Salis and M. Monduzzi, Curr. Opin. Colloid Interface Sci., 2016, 23, 1–9 CrossRef CAS.
- N. C. Stellwagen, A. Bossi, C. Gelfi and P. G. Righetti, Anal. Biochem., 2000, 287, 167–175 CrossRef CAS PubMed.
- M. A. Metrick, J. E. Temple and G. Macdonald, Biophys. Chem., 2013, 184, 29–36 CrossRef CAS PubMed.
- S. O. Ugwu and S. P. Apte, Pharm. Technol., 2004, 86–113 CAS.
- D. S. Katayama, R. Nayar, D. K. Chou, J. J. Valente, J. Cooper, C. S. Henry, D. G. Vander Velde, L. Villarete, C. P. Liu and M. C. Manning, J. Pharm. Sci., 2006, 95, 1212–1226 CrossRef CAS PubMed.
- D. Roberts, R. Keeling, M. Tracka, C. F. van der Walle, S. Uddin, J. Warwicker and R. Curtis, Mol. Pharm., 2015, 12, 179–193 CrossRef CAS PubMed.
- D. Kameoka, E. Masuzaki, T. Ueda and T. Imoto, J. Biochem., 2007, 142, 383–391 CrossRef CAS PubMed.
- L. Y. Chou, H. W. Blanch, J. M. Prausnitz and R. A. Siegel, J. Appl. Polym. Sci., 1992, 45, 1411–1423 CrossRef CAS.
- H. N. Po and N. M. Senozan, J. Chem. Educ., 2001, 78, 1499–1503 CrossRef CAS.
- N. Carlsson, H. Gustafsson, C. Thörn, L. Olsson, K. Holmberg and B. Åkerman, Adv. Colloid Interface Sci., 2014, 205, 339–360 CrossRef CAS PubMed.
- M. Hartmann and X. Kostrov, Chem. Soc. Rev., 2013, 42, 6277–6289 RSC.
- E. Magner, Chem. Soc. Rev., 2013, 42, 6213–6222 RSC.
- M. Piludu, L. Medda, F. Cugia, M. Monduzzi and A. Salis, Langmuir, 2015, 31, 9458–9463 CrossRef CAS PubMed.
- M. Piras, A. Salis, M. Piludu, D. Steri and M. Monduzzi, Chem. Commun., 2011, 47, 7338–7340 RSC.
- J. M. Bolivar, I. Eisl and B. Nidetzky, Catal. Today, 2015, 259, 66–80 CrossRef.
- A. Baeza, M. Colilla and M. Vallet-Regí, Expert Opin. Drug Delivery, 2015, 12, 319–337 CrossRef CAS PubMed.
- A. Salis, L. Medda, F. Cugia and M. Monduzzi, Colloids Surf., B, 2016, 137, 77–90 CrossRef CAS PubMed.
- H. Essa, E. Magner, J. Cooney and B. K. Hodnett, J. Mol. Catal. B: Enzym., 2007, 49, 61–68 CrossRef CAS.
- D. Steri, M. Monduzzi and A. Salis, Microporous Mesoporous Mater., 2013, 170, 164–172 CrossRef CAS.
- A. Salis, M. S. Bhattacharyya and M. Monduzzi, J. Phys. Chem. B, 2010, 114, 7996–8001 CrossRef CAS PubMed.
- V. S. Stoll and J. S. Blanchard, in Methods in Enzymology, 2009, vol. 463, pp. 43–56 Search PubMed.
- D. F. Parsons and A. Salis, J. Chem. Phys., 2015, 142, 134707 CrossRef PubMed.
- J. Meissner, A. Prause, C. Di Tommaso, B. Bharti and G. H. Findenegg, J. Phys. Chem. C, 2015, 119, 2438–2446 CAS.
- M. A. Smith, M. G. Ilasi and A. Zoelle, J. Phys. Chem. C, 2013, 117, 17493–17502 CAS.
- M. Jaroniec and L. A. Solovyov, Langmuir, 2006, 22, 6757–6760 CrossRef CAS PubMed.
- C. Bunton, F. Nome, F. H. Quina and L. S. Romsted, Acc. Chem. Res., 1991, 24, 357–364 CrossRef CAS.
Footnotes |
| † Dedicated to Prof. Barry W. Ninham for his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental details and additional experimental data. See DOI: 10.1039/c6ra17356j |
| This journal is © The Royal Society of Chemistry 2016 |