Recent advances in electrospun metal-oxide nanofiber based interfaces for electrochemical biosensing
Kunal Mondal
*a and
Ashutosh Sharma
*b
aDepartment of Chemical and Biomolecular Engineering, North Carolina State University, 911 Partners Way, Raleigh, North Carolina 27695, USA. E-mail: kmondal@ncsu.edu; Tel: +1-919-260-9449
bDepartment of Chemical Engineering, Indian Institute of Technology Kanpur, Kanpur-208016, Uttar Pradesh, India. E-mail: ashutos@iitk.ac.in; Fax: +91-512-259-0104; Tel: +91-512-259-7026
First published on 20th September 2016
Abstract
The use of unique nanostructured materials has gained substantial importance in the field of biosensing and biomedical applications. Recently, the electrospinning technique has attracted immense attention in the development of nanofiber-based biosensors. Electrospinning has been accepted as a proficient practice for the fabrication of polymer, metal and metal-oxide nanofibers. Electrospinning appears to be the ultimate technique to generate biocompatible and biodegradable polymer/metal-oxide nanofibers, such as carbon, ZnO, TiO2, and NiO, for highly sensitive biosensing applications. Biosensors with enhanced sensitivity are becoming fascinating for the sensing of blood, and in particular for glucose, cholesterol, triglyceride, and low density lipoprotein (LDL) affinity sensing. Electrospinning can deposit a three-dimensional porous nanofibrous mat network on the surface of a sensor transducer, which provides a large global pore volume, predictable pore size distribution, and tunable interconnected porosity. These features of nanofibers can add further suitable functionalities to a sensor for the detection of bio-analytes in a specific environment, where efficient mass transport is needed towards the electrode surface. Electrospun fibers are able to form a highly porous nanofibrous web and their huge surface to volume ratios could lead to very high sensitivity due to their exceptional specific surface areas and interesting nanostructured morphologies, which give rise to properties that do not exist in fibers or wires with a bigger size. Based on the distinctive properties of electrospun nanofibers in the nanoscale that differentiate them from other nanostructures created by other existing methods, we describe in this review the knowledge on nanofibers suitable for biosensor and biomedical applications, including structure and property characterization. Additionally, information on polymers together with metal-oxides precursors and their processing conditions for the electrospinning of ultrafine metal-oxide fibers is briefly described in this paper. Additional relevant issues concerning the research challenges, technology limitations, and future trends are also discussed.
1. Introduction
A biosensor is a device that detects, records, and transmits some information about a physiological change or process for biomolecules.1 An electrochemical biosensor is a sensor that is a self-contained integrated biochip, and provides precise measureable or semi-measurable analytical evidence using a biomolecular recognition element (biochemical receptor) which is involved through a contact with an electrochemical transduction component.2 In the broad outline of a biosensor, the biological recognition element replies towards the target compound and the transducer part changes the biological reaction to a demonstrable signal, which can be quantified in terms of either optics, acoustics, mechanics, calorimetry, electrochemical, or electronic means, and then associated with the concentration of the analyte.3Electrospinning, which is a top-down approach for nanofabrication, has been accepted as an efficient technique for the production of polymer nanofibers.4,5 It is a low cost, versatile and a simple method to fabricate nanofibrous supports.6 Nanofibrous supports display numerous benefits owing to their high porosity and interconnecting pores as compared to other supports such as mesoporous SiO2 nanoparticles and porous alumina.7,8 Several polymers have been efficaciously electrospun in the form of ultrafine fibers using either polymeric blend solutions or in melt form. An extensive range of polymers, which extend from the more “common” polymers, such as PAN9 (polyacrylonitrile) and PMMA10,11 (poly(methyl methacrylate)), to more unusual conducting polymers, such as polyaniline,12 including biopolymers, such as chitosan13 and cellulose,14 have been successfully electrospun using this technique. The commonly used materials produced by electrospinning are polymers, carbon and metal or compatible metal-oxide precursors. PAN and PMMA-based polymers have become a popular material for the modification of surfaces functionalities that are used in biosensing because of their nonspecific surface interaction with biomolecules and electrode materials in aqueous environment.15,16 In addition, several synthetic polymers, such as poly(vinyl alcohol),17 poly(glycolic acid),18 and poly(lactic acid),19 have also been used for biosensors, and biomedical and tissue engineering applications.
The surface properties of these nanofibers can be altered through functionalization, and highly active surface areas can be obtained by easily adjusting nanofiber size, mass and material content. Exceptional nanofiber morphologies and surface textures can be exploited in innovative applications, such as micro/nanofluidics, catalysis and photocatalysis, drug delivery and release, nanoporous supports, energy storage, photon harvesting, nanoelectronics and optoelectronics, reinforcement in various nano and nano-biological composites, gas sensors and electrochemical biosensing.20,21 The nanofiber nanostructure has unique physicochemical, mechanical, optoelectronic and magnetic properties at nanointerfaces which help in superior sensing and offer new openings for the development of highly sensitive biosensor platforms22 and ultrasensitive diagnostic devices.23
Additional requirements for these purposes are essential, such as fast electron transfer, stable redox potential and cycling property of the fabricated structures, which could be obtained by simply choosing the proper precursors during the course of electrospinning. The current research has concentrated on biocompatible and biodegradable scaffold materials for electrochemical biosensing applications that expend naturally occurring polymers such as starch, gelatin, chitosan, collagen and cellulose.24–26 Especially, there have been many efforts for glucose,27 cholesterol,28 triglyceride,29 low density lipoprotein30,31 and uric acid biosensing.32 A distinguished feature of these biosensors is that their physical structures must be in the nano-dimension in order for the transducer materials to be more active. Interestingly, the diameters of electrospun fibers fall into this particular regime and a diameter ranging from several tens to several hundreds of nanometers creates an entangled and covalently-linked network mesh.
Metal-oxide nanofibrous meshes have been projected for futuristic applications in chemical catalysis,33 supercapacitors34 and Li-ion batteries for energy storage,35 electronic,36 magnetorheological usage,37 wastewater treatment,38 gas sensing,39 biosensing,40 tissue engineering41 and biomedical devices42 owing to their interconnected porosity and enormous surface area to volume ratios and other useful properties.43 Several bottom-up and top-down nanofabrication approaches are available for the fabrication of metal-oxide nanofibers, for example vapor–liquid–solid (VLS),44,45 nanocarving46 and electrospinning47–49 have been successfully demonstrated. Amongst them, electrospinning is possibly the most adaptable, since it permits the production of nanofibrous webs from an extensive choice of both organic, inorganic and biological materials.50 Furthermore, electrospinning allows for the control of diameter, surface and pore morphology, orientation, and composition of the resulting metal-oxide fibers.51 Besides solid nanofibers, one can easily fabricate substrate independent co-axial,52 hollow porous53 and free-standing metal-oxide54 nanofibers, which can open up new horizons for applications such as biosensor and biomedical diagnostic devices requiring specific surface functionalities in their nanobio interfaces. The use of calcined electrospun mats obtained from chosen metal oxide precursors is n common tactic owing to its competence and possibility for upscaling. The high temperature calcination method is usually applied to an electrospun polymer/metal-oxide precursor's blend fibers and it is centered on an oxidative change in the polymer constituent by sequential heating in air. There are several effective instances in the literature for the production of metal-oxide nanofibers fibers by the calcinations of suitable precursors synthesized either by the sol–gel process55,56 or from electrospinning of ex situ formed colloidal dispersions.57 At the time of calcination, as the temperatures increases metal-oxide nanocrystals starts to nucleate and grow, whereas the templated polymer is degraded and removed completely after a certain temperate, which further leads to a reasonable decrease in fiber diameter.58,59
Interestingly, since the stability, selectivity, sensitivity, and other analytical features of biosensors are necessary to build an appropriate microenvironment for transferring electrons directly from an enzyme's energetic sites to the working electrode, several conventional materials platforms have been anticipated.60–62 Nanostructured electrospun metal-oxide nanofibers not only preserve the electrocatalytic activity of the immobilized enzyme but also boost the sensing characteristics of enzyme-based biosensors.63 However, many non-oxide semiconductor nanofibers are not very beneficial since their surfaces are unstable in air, which form a nonconductive intrinsic oxide film and may destroy device consistency and intern sensitivity is affected.64 Interestingly, the surfaces of electrospun metal-oxide nanofibers support direct electron transfer and thus augment the analytic signal of the biocatalytic reaction.40 Also, if a redox biomolecule is immobilized on a metal-oxide nanofiber, it shows sensibly faster electron transfer and authorizes the electrochemical surface reactions without the addition of any mediator.63
Owing to their cost effectiveness, specificity, mobility and fast response with high sensitivity, electrospun carbon, metal, metal-oxide and composite nanofiber based electrochemical biosensors are expected to perform a crucial role in clinical (cancer detection65 and diagnostics of infectious organisms66) and non-clinical (numerous food toxins67 and genetically altered organisms68) point-of-care diagnostic device69,70 and environmental monitoring (detection of pesticides,71 heavy metal ion traces,72 pollutants73 and genotoxic particles74) applications. Herein, we report in this review various amperometric and voltammetric electrochemical enzymatic and enzyme-free biosensor platforms based on electrospun metal-oxide nanofibers for economical, highly sensitive, stable, wide range and fast sensing of biomolecules for various biosensing and biomedical applications.
2. Electrospinning to fabricate nanofibers
2.1 Solid nanofibers
Electrospinning is a top down approach for the synthesis of nanomaterials in the form of nanofibers.75 The fabrication of nanofibers by electrospinning has gained much research and industrial attention in recent years.76 Polymer and metal oxide nanofibers demonstrate some interesting belongings that make them promising for several applications. Nanofibers have a huge surface area to volume ratio, super flexibility in surface functionalities, and mechanical properties superior to bigger diameter fibers.77 Some applications worth mentioning for nanofibers include biocompatible scaffolds tissue engineering,78 devices for nanofiltration,79 sensors for chemical80 and biological81 uses, and several electronic,82 actuator83 and energy storage devices.84In the standard electrospinning procedure, fibers are spun from a solution or melt through a metallic needle by applying a high electric field. Electrospun nanofibers are normally collected as randomly oriented unaligned or partially-aligned fibrous mats. Unaligned randomly oriented fiber (Fig. 1a) mats result when spinning is done on a static collector,85 and partially-aligned mats are collected when a rotating collector (Fig. 1b) is used.86 The electrospinning setup can also be modified so that resultant nanofibers can be collected between two parallel electrodes.87 Since single nanofibers can be collected by this technique, which magnifies the possible uses of electrospinning. After the as-spun fibers are collected they may be subjected to further processing to create configurations appropriate for particular applications. Sharma and coworkers88 collected electrospun fibers between two SU8 polymer derived conductive carbon micro posts and measured the conductivity of the single fibers. Li et al.89 collected electrospun PVP (poly(vinylpyrrolidone)) nanofibers between two conductive silicone posts, whereas Teo et al.90 electrospun fibers across two tiny metallic blades. In this context, Parker's group91 developed a simplistic way to manufacture three-dimensional aligned nanofibrous webs by employing high-speed rotating polymer solution jets.
 | ||
| Fig. 1 (a) Electrospinning setup for randomly oriented nanofibers, and (b) rotating mandrel for partially align nanofibers. | ||
The diameter of electrospun nanofibers can be varied from a minimum of 10 nm to a few microns and can be adjusted methodically by simply altering electrospinning parameters such as polymer concentration, applied electric field and flow rate of the feed solution.92 The length of the fibers depends on these parameters and the geometry and size of the collector mandrel.93 Thus, the electrospinning process should be optimized based on the aforementioned parameters to obtain uniform, long, continuous fibers of a desired orientation, and diameter.
2.2 Effect of electrospinning parameters on spun nanofibers
Polymer concentration is the most significant parameter that controls the fiber diameter and length during electrospinning.95 The length and diameter of the fibers increase with an increase in polymer concentration at a fixed electric field and feed rate. At a fixed solution feed rate and constant voltage, the reduction in fiber diameter is proportional to the decrease in the polymer solution concentration until it reaches a critical concentration, below which disconnected polymer beads are formed rather than continuous fibers. Superior quality, uniform diameter fibers without any beads are achieved at an optimized polymer concentration. A higher polymer concentration gives larger diameter rough fibers. At high polymer concentration, sometimes the formation of fibers is hampered because of the high viscosity of the polymeric solution. For example, Sharma's group reported94 that in a PVP solution, polymer concentration is the most significant parameter to control the fiber diameter (the effect of the parameter is described in Fig. 2). In Fig. 3, the FESEM micrographs (Fig. 3a–c) describe a particular case where a polyacrylonitrile/zinc acetate (PAN/ZnAc) blend is electrospun on a rotating mandrel. | ||
| Fig. 2 The effect of the electrospinning process parameters on fiber diameter. Reprinted with permission from ref. 94. Copyright (2014) American Chemical Society. | ||
 | ||
| Fig. 3 FE-SEM images describing the variation of fiber morphology of electrospun PAN/ZnAc nanofibers with varying PAN polymer concentrations while other parameters are fixed. (a) Appearance of more beads and non-uniform diameter fibers at 4 wt% concentration; (b) uniform diameter of the fibers at 8 wt% concentration and (c) larger fiber diameter at 12 wt% concentration. Reprinted from ref. 38, Copyright (2012), with permission from Elsevier. | ||
The solution feed rate is another parameter to control fiber diameter.96 As soon as the solution feed rate is adequate to form fibers, a further increase in flow rate hardly matters to morphology. A higher solution feed rate only delivers more polymer solution than actual needed. It has been observed that the excess amount polymer of solution forms beaded thick fibers and any further increase in feed rate clogs the needle tip. A higher feed rate gives a very short drying time to the polymer prior to reaching the collector surface and is responsible for low stretching forces. Usually, a lower flow rate is preferable (as shown in Fig. 2) because the polymer solution will acquire adequate time for polarization, however very low flow can cause an insufficient supply of polymer feed. Therefore an optimized feed rate is a must for the sufficient production of good quality fibers. For example, Fig. 4a and b describe the variation of effect of electrospun fiber diameter with varying solution feed rates. Although a few reports92,97 say that it has a minute effect or no significance on the length, uniformity and diameter of the fibers, other researchers94,96,98 have different views.
 | ||
| Fig. 4 FESEM micrographs showing a the fiber morphologies of an 8 wt% PAN/ZnAc solution electrospun at a solution feed rate of (a) 20 μL min−1, and (b) 120 μL min−1. Reprinted from ref. 38, Copyright (2012), with permission from Elsevier. | ||
Applied voltage is another key parameter for the electrospinning process.99 A threshold voltage is necessary to overcome the surface tension force of the polymer and breakage of the form of the thin jet composed of micro droplets.100 The charge jet forms a Taylor cone101 and a high electrostatic charge is formed in the polymer which drives the jet towards the grounded collector surface to form the nanofibrous web. If the electric field is also varied, keeping other parameters fixed, at a lower electric field strength, non-uniformity in fiber diameter and very low production rate is found. At a very high electric field strength a nonuniform fiber morphology, thicker fiber diameter and the existence of micro-droplets on the fibers are observed. Hence, a middle range of field strength should be chosen to get optimum nanofiber mats. However, a few researchers claim that there is no considerable influence of electric field strength on the diameter of electrospun nanofibers.102 Some groups also recommend that a higher electric field can intensify the electrostatic repulsive force on the charged polymeric jet which could favor the thinning of the fiber diameter.103 Therefore, the electric field may have an impact on the diameter of the fibers, however the level of consequences differs with the concentration of the polymer solution and the other process parameters.
There are a few other parameters, such as the nature of the collector, the distance between the syringe tip and collector, that need to be optimized to obtain better quality fibers. Additionally, ambient parameters, such as humidity and temperature, can also interrupt the fiber diameter and morphology. It has been proven that the growing temperature favors thinner fibers due to the counter correlation between the polymer solution viscosity and temperature.104 In the case of humidity, low humidity increases the solvent evaporation rate and can dry the solvent fully. On the other hand, elevated humidity leads to a large fiber diameter since the electric charges on the jet can be counteracted and thus the stretching forces become insignificant. Recently, Casper et al. confirmed that a change in humidity affects the diameter and surface morphologies of electrospun fibers.105
The coaxial electrospinning technique is mostly reported as the most versatile method for the fabrication of hollow nanofibers.111 The functional properties on the surface of the nanofibers are imposed via the shell material, while the inherent properties of the nanofibers are in the core. The concept of co-axial or core–shell nanofiber nanostructures can be adapted.112 Here, in this special nanostructure, the outer layer may be comprised of active functional materials to introduce further functional properties, for example shells carrying immobilized specific bio enzymes.108 In general, hollow nanofibers are produced with this method when the core material is used as a sacrificial polymer and subjected to removal by either post heat treatment or by chemical etching.
In a typical spinning process (shown in Fig. 5), a coaxial polymeric jet is designed by a coaxial spinneret where two dissimilar polymers or liquids run through the inner (core) and outer (shell) capillaries concurrently. Both of the capillaries are linked to a high voltage source (∼10 kV) and nanofibers are formed during solvent evaporation and stretching. The flow rate ratio of the two feeding solutions is an important parameter for the uniformity and stability of the polymeric jet in the core. The thickness of the nanofiber shell can also be tuned by adjusting the flow rates of the inner and outer liquids in the capillaries in the coaxial electrospinning process. Additional factors, such as the dimension of the core–shell capillaries, electric field strength, volumetric feed rate, and non-miscible nature of core–shell fluids and their viscosities along with conductivities, also show a vital role in the uniform flow of core–shell jets, which affects the production and morphology of the core–shell electrospun nanofibers.113 Usually, the polymer with lower surface tension is driven through the external capillary (i.e., shell). For instance, in the case of the polystyrene and polyaniline system, polystyrene is used as the shell polymer on polyaniline because of its poorer surface tension.114 In the same way, for polyacrylonitrile polymer derived hollow carbon nanofibers by coaxial electrospinning, poly(methyl methacrylate) is used as the pyrolytic core precursor and polyacrylonitrile is used in the shell as the carbon precursor.115
In general, the calcination of a metal-oxide precursor derived electrospun nanofibrous mat is a frequently used route because of its competence and prospective for mass production. The calcination process is based on two aspects, which include polymeric phase removal by burning at high temperature in presence of oxygen and the oxidative conversion of the precursor component to produce the metal-oxide by high temperature nucleation and growth.120,121
Interestingly, metal-oxides can be introduced into the electrospun fibers in situ or by ex situ in the form of a surface coating on a polymeric and/or carbon core. In the first case, an appropriate sol–gel or organometallic metal-oxide precursor is mixed with the electrospinning polymer solution, whereas co-axial electrospinning or just dipping polymer nanofibers into a solvent bath containing the desired precursor produces a surface coating of metal-oxides. There are several positive instances in the literature for the fabrication of metal-oxide nanofibers by the calcination of precursor materials prepared either by sol–gel routes,38,40,55,69,94,122–124 or electrospinning of polymeric colloidal dispersions formed ex situ.125–129 In the case of the electrospinning of an inorganic precursor, calcination induces shrinkage of the fiber diameter almost instantaneously since the carrier polymer template is degraded. Subsequently, due to calcination, the nanofibers generally become more brittle due to their thinner cross section and the thermal and internal mechanical stress generated through the shrinkage in size.40,130 Hence, it is desirable for a supplementary material, which is chemically and mechanically unaffected by heating during the course of calcination, to be combined or impregnated in the electrospun nanofibers during electrospinning or incorporated before heating. There have been many attempts recently for the fabrication of metal or metal-oxide nanofibers by employing the electrospinning technique. For example, zinc, silica126,128 and titania131 nanoparticles were incorporated in polymer fibers by electrospinning a blend of polymers and dispersions of these nanoparticles. In addition to this, Zn based metal-oxide precursors or inorganic zinc salts have been mixed with a solvent compatible carrier polymer to produce electrospun ZnO nanofibers.38,132 In recent times, the electrospinning of zinc and titanium dioxide bi-component metal-oxides133 and metal–metal-oxide (silver/ZnO)134 nanofibers have also been reported. Horzum et al.117 demonstrated the fabrication of metal oxide/silica nanofibers by employing the colloid electrospinning technique. Recently, Sharma's group40,94 used titanium isopropoxide, which is a sol–gel precursor of titanium dioxide, along with the templating polymer, PVP, to produce electrospun carbon doped TiO2 nanofibers. The electrospun nanofiber mats were dried in air to complete the hydrolysis of the titanium dioxide precursor and then calcined in air to yield mesoporous TiO2 fibers. The calcination of the spun fibers removed the PVP polymer and produced mesopores which play an important role in highly sensitive electrochemical biosensing applications. Also, the resultant TiO2 nanofibers can be doped with carbon by simply adjusting the calcination temperature and time of the fibrous mat.94
However, there are other materials which can also be incorporated into electrospun fibers such as metals and ceramic, DNA. For example, metal nanoparticles can be easily incorporated into the nanofiber matrix. In the recent past, Wu et al.135 fabricated copper nanofibers for high-performance transparent electrodes by electrospinning. Kim's group prepared pure metallic nickel nanofibers using the electrospinning technique.136 Interestingly, thin fibers of calf thymus Na-DNA were also electrospun from aqueous solutions by Reneker's group.137 In this context, Schiffman et al.138 published a wonderful review article on electrospun biopolymeric nanofibers, which explains their useful applications.
3. Application of electrospun metal-oxide nanofiber based interfaces in electrochemical biosensors
3.1 Basics of the electrochemical biosensing platform
A sensor is a practical device that records a chemical, biological or physical alteration and alters that into a quantifiable signal.143,144 The sensor (shown in Fig. 7) is comprised of a recognition component, which allows selective reaction to a specific analyte or an assembly of analytes, and accordingly reduces interferences from other sample modules. Another central module of a sensor is the transducer or the detector which harvests a signal and delivers it to a digital signal processor. A signal processor collects the signal and then amplifies by its internal electronics, and exhibits the signal on an electronic display. Electrochemical biosensors fall into the subcategory of chemical sensors that associates sensitivity, which is specified by the low detection limits of electrochemical transducers, with the superior specificity of biological recognition procedures. These electrochemical biosensors are comprise of a biological recognition element (which may be proteins, enzymes, cells, tissues, antibodies, receptors, or nucleic acids) that reacts with the target analyte very selectively and thereby creates a signal in the form of an electrical current, which is associated to the targeted analyte concentration. Electrochemical biosensors can be categorized into two leading classes depending on the environment of the biological recognition process, which are namely affinity sensors and biocatalytic devices. A biocatalytic device incorporates enzymes, entire cells or tissue which can recognize the target analyte and yield electroactive species. On the other hand an affinity biosensor depends on a specific binding interface among biomolecules, such as antibodies, and the targeted analyte.3.2 Electrochemical detection
In a biosensor the sensing of electrical properties for acquiring information from biological schemes is usually performed electrochemically, where the bioelectrochemical part works as the central transduction element. Although biosensor devices have engaged different varieties of recognition elements, the electro-chemical detection methods work mainly with enzymes. This is typically because of their specific binding abilities and biocatalytic activities.143,145–147 Other biorecognition elements, such as antibodies, nucleic acids, whole cells or cell fragments and micro-organisms, are also used in electrochemical detection. Also, an immunosensor uses antibodies, or antigens to observe the binding procedures in electrochemical biosensing reactions. Typically in an electrochemical biosensor, the electro-bio-catalytic reaction either produces a quantifiable current (amperometric sensing), a measurable electric potential or charge buildup (potentiometric sensing), or detectably modifies the electrical conductivity of a medium (conductometric sensing) between bio-electrodes.148 There are also other types of electrochemical detection systems, for example impedimetric biosensors, which deal with impedance for both reactance and resistance,149,150 and field-effect biosensors, which use an electronic transistor to detect current as a consequence of a potentiometric outcome at the surface of a gate electrode.1513.3 Electrochemical biosensing mechanism
Mostly, the bio-chemical reactions in a senor are sensed only in an adjacent vicinity to the active electrode surface where the electrodes themselves play a vital role in the overall performance of the electrochemical system. It is very important to note that detection for a chosen specific function of an electrode strongly depends on its material properties, surface modification and dimensions. Typical electrochemical sensing generally involves a reference electrode, an auxiliary or a counter electrode and a working electrode or redox electrode which is also known the sensing electrode. The reference electrode is usually made from Ag/AgCl or glassy carbon material. A certain distance is maintained between the reference electrode and the reaction location so that a known steady potential could be maintained. The counter electrode is usually made of a platinum wire. This electrode forms an ionic connection to the working electrode through the electrolyte solution so that a current can be ensured between the working and counter electrodes.The role of the working electrode is to serve as a transduction element in the biochemical reaction. The counter and working electrodes should both be conductive and chemically stable. Also, the surface area of the working electrode is important for efficient electrochemical biosensing and for this purpose it is sometimes modified with functional nanomaterials, such as graphene, carbon nanotubes, metal and metal-oxide nanomaterials or polymeric nanostructures, depending on the targeted analyte.147,152,153 An outstanding summary of electrochemical biosensors based on nanostructured metal-oxides is provided in the review by Solanki et al.154 Also, Mehrvar et al.155 wonderfully described the characteristics of electrochemical biosensors in terms of varying detection limits in their work.
For example, Zhou et al.172 developed three-dimensional (3D) porous metal-oxide (ZnO–CuO) hierarchical nanofibrous electrodes for nonenzymatic glucose sensing and reported a good stability and high sensitivity of 3066.4 μA mM−1 cm−2 as compared to other electrodes.172 Ding et al.173 constructed an enzyme-free glucose sensor based on electrospun metal-oxide nanofibers (Co3O4 nanofibers) and showed that the sensor has a fast response time (∼7 s), good sensitivity of 36.25 μA mM−1 cm−2, decent reproducibility, good selectivity, and a detection limit of 0.97 μM. Rahman et al.174 explored the applicability of different nanostructured metal-oxides, including ZnO, CuO (both Cu(I) and (II) oxides), MnO2, TiO2, Fe3O4, CeO2, SiO2 and ZrO2, for the development of glucose based biosensors. Recently, Chen and coworkers beautifully described the recent progress, perspectives and current challenges of nonenzymatic electrochemical sensors with hundreds of references.175 The electroanalysis and electrocatalysis of nickel and its oxides, hydroxides and oxyhydroxides for small molecules has been discussed broadly by Miao et al.176 with over eighty references in their recent review article.
It is very essential to understand electrochemical enzymatic biosensors, in which one has to concentrate on the process of electrical communication between the enzyme active site and the bioelectrode via an artificial redox mediator. In the course of this electro catalytic reaction, the enzyme either engenders or puts away a redox-active species (i.e., bio active charge carrier) at the time of conversion of the target analyte molecules. The bioactive compounds are oxidized via the enzymes and produce electrons which charge the transducer element at the electrode interface to produce an electrical potential that matches up to the expanse of oxidized analyte (shown in Fig. 8). The most common category of redox mediators comprises ferrocene molecules and their end product depending on their huge potential window to power a redox reaction. The request of a mediator based system permits much less operational potential to control the catalytic reaction since mediator molecules are employed to move electrons between the enzyme's redox reaction point and surface of the electrode.
3.4 Enzyme immobilization on electrospun metal-oxide nanofiber interfaces
To create a sustainable biosensing platform, the enzyme has to be correctly grafted to the transducing material with sustained biological efficiency. This method is called enzyme immobilization. Fig. 9a and b show the enzyme immobilized surfaces of electrospun metal-oxide TiO2 nanofibers, and Fig. 9c and d describe the crystalline morphology of the nanofibers. In recent times, enzyme immobilization onto nanostructured material surfaces has achieved strong attention because of the unique features of the resulting assemblies. Enzymes are well-known for their high degree of specificity which gives them a high efficacy for device based applications in protein design, medicine, biomedical and biosensing. Interestingly, their dependency on the electrocatalytic activity of the surrounding micro environment, instability, degradation, easy inactivation, denaturation, inhibition and non-reusability hinder them from global usage in biosensing. Customarily, biosensors are fabricated with a huge enzyme loading to ensure that sufficient bioelectrocatalysis occurs. The enzymes are delivered with a proper microenvironment so that they could withstand their bioactivities. The confined thermochemical environment also shows intense influence on the stability of the enzyme. The choice of immobilization technique is influenced by several factors, for example, the origin of the biological element, the nature of the transducer material, the physical and chemical characteristics of the analyte, and the working environments where the biosensor has to perform. Considering all these concerns it is crucial for the biological element to show supreme activity in its enzyme immobilized environment. | ||
| Fig. 9 FE-SEM and TEM micrographs showing the cholesterol esterase and cholesterol oxidase enzymes immobilized on electrospun TiO2 nanofibers. (a) Image of the enzyme immobilized nanofibers and (b) high resolution image. (c) TEM micrograph of calcined TiO2 nanofibers, with the pores visible in the inset and (d) SAED (selected area electron diffraction) pattern of the electrospun TiO2 nanofibers. Reprinted with permission from ref. 40. Copyright (2014) American Chemical Society. | ||
In the case of an enzyme-based biosensor, a biochemical reaction happens between the analyte and a biocatalyst immobilized onto a substrate. Normally, there are four usual methods for enzyme immobilization, namely, adsorption, entrapment or encapsulation, covalent bonding and cross-linking.177,178
In recent years, there have been a few reviews that envisage the development of enzymatic electrochemical biosensors based on numerous materials, methods, and practical applications. Erden et al.186 described the development of amperometric uric acid biosensors over the past two decades covering sensing mechanisms, enzyme immobilization, electrode materials along with their benefits and limitations. Chen et al.187 nicely described the recent advancements in electrochemical glucose detection, sensing principles, methods and recent developments, problems and bottlenecks in the areas of enzymatic (glucose oxidase-based) and enzyme-free amperometric glucose sensing. Ispas et al.188 systematically discussed the recent developments in enzymatic biosensing for biomedical analysis including microfluidic biosensing, development of new technologies and a few innovative designs, such as paper based-sensors, biochips, lab-on-a-chip. Cipolatti's group focused on the current status and some trends in enzyme-based immobilization techniques on nanostructured surfaces.189 Malhotra's group highlighted in their review the recent progress in various materials and sensing techniques for cholesterol biosensors.190 The exclusive electrocatalytic activities of metal-oxides for electrochemical enzymatic biosensor applications have been discussed by Ansari et al. in their recent review.191 A broad and critical review on metal-oxide nanoparticle based enzymatic biosensors, their growth and future outlooks have been well described by Shi et al.192 It is very important to note that the recent success in this area has increased interest on the expansion of novel enzyme immobilization approaches and direct electron transfer with the help of functional nanostructured materials, especially metal and metal-oxide nanostructures. For instance, Sharma's group recently developed surface modified aligned porous electrospun TiO2 nanofiber for enzymatic esterified cholesterol biosensing.40 Senthamizhan et al.193 presented a review with emphasis on the current progress in the use of electrospun polymers, metal and metal-oxide nanofibers and their composites for glucose biosensors.
3.5 Essentials qualities of nanostructured metal-oxides for electrochemical biosensing
Metal-oxide nanomaterials have incomparable electro-optical, magnetic and electronic properties which make them important candidates for immobilizing biomolecules. For effective immobilization, a narrow particle size distribution is necessary and this can, in principle, be achieved in metal-oxides by controlling the process parameters.194 Additionally, metal-oxide nanomaterials possess very high accessible surface areas over volume ratios, phonon and electron confinement, improved work function, elevated surface reaction, good catalytic activity and robust adsorption capacity via interconnected porous network structures which aid the ultra-high loading of biomolecules on the particle surfaces. It has been demonstrated that phonon confinement in nanowire like structures helps bandgap engineering and can ease charge carrier transport.195,196 The confinement of the electron–phonon interaction in nanostructures has impacts on their luminescence and helps in a variety of immunosensing applications.197 Phonon confinement enhances the thermal conductivity in nanofibers and thus covalent bonding and electrostatic interaction are improved, which aid in better biomolecule loading.198Moreover, metal-oxide semiconductor nanofibers have greatly porous crystalline structures which provide friendly environments during electrochemical reactions to metal ions and provide bridging organic ligands that are linked together by coordination bonds owing to the high accessible surface areas that are facilitated by their tunable interconnected nanoporous cavities.199
Nanostructured metal-oxides open up the possibility of developing new transducer abilities in electrochemical biosensing in the submicron to nanoscale size length scale.174 An electrochemical biosensor integrates a biological element (enzyme, antibody, receptor protein, whole cells or sections of tissue and nucleic acid, etc.) as a sensing element to a transducer material and transmits a signal. Many studies have been conducted involving the effect of the optical-electronic and electrochemical properties of metal-oxide nanostructures for their use in suspended media, and surface activities at nanobioelectrode interfaces for biosensing uses. The faster electron transfer rate depends on the nature of the interaction between the metal-oxide and biomolecules which can further increase the biosensing response signal.200 It is essential to select a nanostructured metal-oxide platform that is appropriate for immobilization of the targeted biomolecules on its surface (Fig. 11 schematically describes electrochemical biosensing by the immobilization of an enzyme on metal-oxide nanofiber surfaces). The binding of a biomolecule and metal-oxide creates a nano-biointerface which is recognized to affect the overall sensing performance of a biosensor. The formation and electrochemical properties of the nanobiointerfaces are regulated by the microenvironment and physicochemical nature of the selected metal-oxide. Other factors, such as the total accessible active surface area, total charge on the surface, surface energy, surface textures, porosity and pore volume, position of valence/conduction band, functional groups, and physical shapes and sizes, all have an impact on the formation of the interface. Generally, physical adsorption or physicochemical forces are the factors that bind biomolecules with nanostructure metal-oxides. Physical adsorption occurs because of weak interaction forces, such as van der Waals and/or electrostatic forces, which mainly depend on the active area and morphology of the surface, medium of the reaction and total charge lying on the surface of a biomolecule. The short-range forces come from charge interactions, steric effects, and interactions of solvents which also help in the formation of an interface between the biomolecule and nanoparticles at the micro/nanoscale. Covalent interactions between a biomolecule and a metal-oxide surface rely on the accessibility of the functional groups, which can be organized by appropriate surface modification via chemical routes. An efficient metal-oxide interface can support biomolecules to preserve their biocatalytic activities with good stability by creating a biocompatible micro/nano environment. Therefore there is a significant opportunity towards electrochemical biosensor development with better sensitivity, improved detection limits, cost-effectiveness and prolonged shelf life, by applying various metal-oxides nanostructured materials. Recently, Malhotra's group discussed this issue very well in their review article.154 The development of various nanostructured metal-oxides, such as ZnO, MnO2, and TiO2 based glucose biosensors, has been covered by Rahman et al.174 It is important to note that various biomolecules, such as enzymes, antibodies, nucleic acids, and cells, can be immobilized easily on electrospun metal-oxide nanofibers owing to their fibrous morphologies at the nanoscale. Recently, Ding et al.201 summarized the progress in the design of highly sensitive and selective nanosensors based on electrospun nanomaterials, and discussed the inherent fundamentals and design optimizations. Ramakrishna et al.202 reported the use of electrospun nanofibers towards environmental engineering and biotechnological applications. Stafiniak et al.203 reported the design of biosensor platforms based on electrospun zinc oxide nanofibers. Recently, a glucose biosensor was constructed by graphene oxide, NiO nanofibers and Nafion modified glassy carbon electrode and the sensor exhibited high sensitivity (1100 μA mM−1 cm−2), quick response time (∼5 s), low detection limit of 0.77 μM, extensive stability, and exceptional anti-fouling capacity for enzyme-free glucose sensing.204
Nanostructured electrospun metal-oxide nanofibers have a tremendous role in interfacing enzymes with signal transduction since the new class of biosensor platforms have active enzymes sites which are directly attached with nanostructured metal-oxide electrodes, thus resulting in effective biocatalysts for efficient electrochemical biosensing. Nanofibers have an easily accessible continuous electronic conduction path which helps direct electron transmission between the enzyme and metal-oxide and further improves biosensing characteristics.174 Also, nanofibrous electrodes offer an electroactive biocompatible surface which allows improved enzyme immobilization with better conformation, alignment and biological efficacy.174 Additional advantages could be obtained by altering the size, morphology and collection of metal-oxide nanofibers using the electrospinning process. For example, electrospun metal-oxide nanofibers can be collected in the form of a free-standing mat without any additional substrate, which can be directly used for support free enzyme immobilization and as a transducing material for efficient electrochemical biosensing. Recently, Liu et al.205 produced electrospun carbon nanofibers and demonstrated that a free-standing nanofiber mat is an ideal platform for electrochemical measurements due to its substrate independent free-standing configuration and low background noise.
A key challenge in the development of bioelectrodes for electrochemical biosensing is the formation of adequate electrical contact between the active sites of enzymes and metal-oxide electrodes. For instance, the redox centers are electrically shielded by protein shells. As an end result, enzymes cannot be reduced or oxidized at the surface of the electrode at all applied potentials. The risk of hindrance in straight electron transfer between the metal-oxide electrode and enzymes may possibly block the development of reagent-free biosensing platforms. Retaining a minimum separation between a redox site and electrode is sometimes very challenging without providing a huge over potential which causes poisoning of the active electrode surface and thus denaturation of biomaterials. Progress in attaining a direct electron transfer path could be made either by altering the enzyme or surface modification of the electrode by introducing a mediator or nanoparticles.
4. Typical electrospun metal-oxide based electrochemical biosensing platforms
Electrospinning is the simplest technique for the extensive large-scale production of polymer, and metal or metal-oxide nanofibers. A noteworthy usage of these nanofibers is to show their prospective in chemical sensing or electrochemical biosensing, which benefits from their nano scale size, ultra-high surface to volume ratios and extraordinary aspect ratios. There are numerous reports in the literature for the successful fabrication of electrospun nanofibers, such as ZnO,38 TiO2,94 AgO,206 Al2O3,207 CuO,208 MnO2,209 ZrO2,210 SnO2,211 SiO2,212 NiO,213 Co3O4,214 Fe2O3 (ref. 215) and composite nanofibers of Cu,208 Ag,216 Au,217 Pt,218 carbon,219 CNT,88 and graphene oxide,220 for biosensing, solar energy harvesting, environmental remediation, catalysis, and energy storage applications. To date, various electrospun metal/metal-oxides, carbon and composite nanofibers have been successfully demonstrated for biosensor device based applications.40 Consistent and faster detection of glucose based on electrospun nanofibers is one of the most explored applications in the field of clinical diagnostics, biotechnology, and food industry.221 Several other biosensors, such as cholesterol, catechol, urea, low density lipoprotein (LDL), triglyceride, dopamine, and cancer biomarker detection platforms, have been reported to work efficiently using electrospun nanofibers.201,222 Here, we have discussed a few common metal-oxide nanostructures fabricated by the electrospinning technique, which are mostly used in electrochemical biosensing platforms.4.1 Electrospun ZnO nanofiber based biosensing platforms
Electrospun ZnO nanofibers have an extensive prospective in various applications with nanoelectronics, optoelectronics and sensing devices. Nanostructures of ZnO could be of distinct research value in biosensors and bioelectronics applications since they have relatively low bio-toxicity, are transparent and have a direct band gap and big excitonic binding energy. ZnO is an n-type metal-oxide semiconductor with oxygen vacancies and interstitial Zn availability. The fibrous nanostructured surfaces of electrospun ZnO show a noteworthy character in defining its conductivity and continuous electron transfer through the fiber, and thus it is a useful candidate for transistor applications. Even though ZnO based nanomorphologies have been employed extensively for optical, humidity and gas sensing, their excellent biocompatibility and superior isoelectric point of about 9.5 make them appropriate materials for use in enzymatic electrochemical sensors.223 The absorption of enzymes onto ZnO surfaces with low IEPs is most desirable since enzyme immobilization is mainly driven by electrostatic covalent interaction. Additionally, ZnO nanofibers could offer an appropriate microenvironment for enzyme immobilization and not hamper bioactivity, and as a result open up an extended application for the development of electrochemical biosensing platforms with improved bioanalytical performance. ZnO-based nanofiber nanostructures have been explored for enzyme-based electrochemical biosensing of various target biochemical analytes such as glucose, cholesterol, LDL, H2O2, phenol, uric acid, triglyceride and dopamine. To date, several works have reported glucose sensors based on electrospun ZnO nanofibers. For example, Ahmad et al.224 successfully demonstrated an amperometric glucose biosensor which was constructed by a single electrospun ZnO nanofiber. First, a polyvinylpyrrolidone (PVP)/zinc acetate blend was electrospun followed by calcination at a high-temperature, which produced ZnO nanofibers with fiber diameters in the range of 195–350 nm. Then, a single electrospun ZnO nanofiber was shifted on a gold electrode and subsequently immobilized with glucose oxidase (GOx) through physical adsorption. The KM value (2.19 mM) shows efficient GOx immobilization with superior enzymatic activity. Their electrochemical biosensing measurements report that the sensor has the highly reproducible sensitivity of ∼70.2 μA cm−2 mM−1 in a linear experimental range of 0.25–19 mM with a low limit (1 μM), fast response time (<4 s) of detection and favorable stability of more than 4 months. Wu and coworkers225 developed an electrochemical sensor to detect dopamine based on phosphotungstic acid–ZnO electrospun nanofibers on a platinum electrode. The modified working electrode exhibited exceptional electrocatalytic activity towards the oxidation of dopamine in phosphate buffer solution owing to the high active surface of the ZnO nanofibers. The biosensor shows good sensing with a detection limit of 0.089 μM within a dopamine concentration range of 0.19 μM to 0.1 mM at pH 5.Recently, Sharma's group69 developed a point-of-care immunosensor device (Fig. 12 describes the scheme for the working idea of the sensor) for label-free breast cancer biomarker (anti-ErbB2; epidermal growth factor receptor 2) detection based on mesoporous electrospun ZnO nanofibers with a diameter in the range of ∼50–150 nm. They claimed that the biosensing platform is more sensitive than the best confirmed in the literature and much more sensitive than the available ELISA standard for the detection of the breast cancer bio-marker. This biosensor is proven to be efficient, highly selective and extremely reproducible with extraordinary femto-molar sensitivity to detect early stage breast cancer. In their work free-standing mesoporous ZnO nanofiber mats were prepared by electrospinning a polyacrylonitrile/zinc acetate blend followed by calcination at a controlled temperature. The diameter and morphology of the electrospun nanofibers were controlled by optimizing the electrospinning parameters, specifically the concentration of the polymer/precursor blend, applied voltage and the rate of the solution feed. Fig. 13a–c show FESEM micrographs of the free-standing ZnO nanofiber web and the nanofibers after calcination at low and high magnification, respectively. The working electrodes were prepared by coating a thin film of ZnO nanofibers which were deposited by electrophoretic deposition on ITO (indium tin oxide) glass. Then, the anti-ErbB2 breast cancer biomarker was immobilized onto the electrospun ZnO fibers via covalent interaction (Fig. 13d and e show FESEM images of anti-ErbB2 immobilized onto ZnO fibers, while Fig. 13f shows a TEM micrograph of the ZnO nanofibers and confirms its crystallinity). They also revealed that oxy-plasma treatment produces –COOH, –OH, etc. functional groups which further help in anti-ErbB2 immobilization on the electrode surface. This label-free detection was achieved by the electrochemical impedance technique which can detect up to a 1 fM biomarker concentration with a stable high sensitivity of 7.76 kΩ μM−1. Sharma demonstrated that this electrochemical immunosensor can work in an extensive detection test range of 1.0 fM to 0.5 μM and can offer quick detection (128 s). The plasma treated bio-electrode has a large association constant of 404.8 kM−1 s−1, which signifies good affinity to theErbB2 antigen.
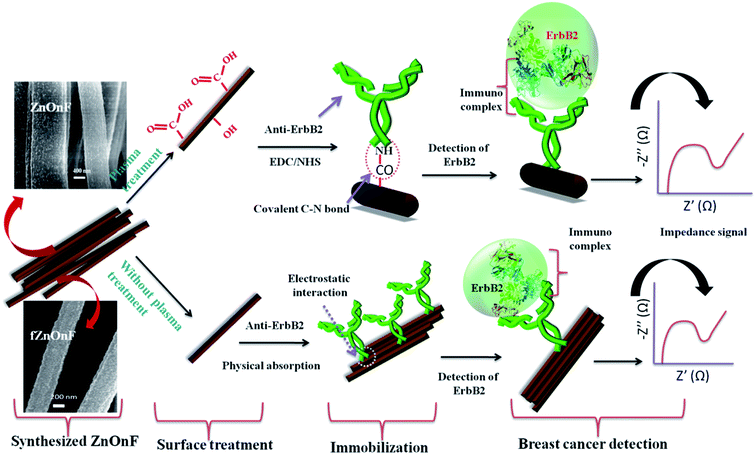 | ||
| Fig. 12 Schematic illustration of the fabricated immunosensor for label-free breast cancer biomarker detection. Reproduced with permission from ref. 69. Copyright 2015, Royal Society of Chemistry. | ||
 | ||
| Fig. 13 FE-SEM and TEM micrographs of calcined electrospun ZnO nanofibers. A free-standing calcined ZnO nanofiber mat at (a) low magnification, (b) high magnification, and (c) distinct ZnO nanofibers in high resolution. FESEM image for ErbB2 anti-body loaded ZnO nanofiber surfaces at (d) low and (e) high magnification. (f) TEM micrograph of a single ZnO nanofiber with its individual grains shown in the inset. Reproduced with permission from ref. 69. Copyright 2015, Royal Society of Chemistry. | ||
In a recent study, a uric acid biosensing device based on a carbon paste electrode modified by electrospun ZnO nanofibers and quercetin was demonstrated by Arvand et al.226 The fabricated composite electrode was able to determine uric acid traces in blood serum and plasma of a healthy human as well as in a leukemia patient. This electrochemical sensor has a detection limit of 0.05 μM L−1 with an RSD = 0.2% for uric acid sensing.
4.2 Electrospun TiO2 nanofiber based biosensors
Titanium dioxide nanofibers are one of the distinctive biocompatible nanostructured materials that have been extensively used in the biotechnology and biomedical engineering fields because they are strong oxidizing agents, chemical inert and nontoxic. Electrospun TiO2 nanofibers are moreover environmentally green and have commonly been projected as a promising interface for enzyme immobilization, and broadly applied in catalysis, photocatalysis, and electrochemistry. Fortunately, TiO2 nanofibers retain the bioelectrocatalytic efficiencies of the immobilized enzymes due to the fact that Ti can create covalent bonds with enzymes amine and carboxyl groups. Nanostructured electrospun TiO2 also offers a higher surface area to volume ratio and porous morphology, and in this way affords an enhanced immobilization microenvironment to enzymes.In this context, Tang et al.227 fabricated an enzyme-based glucose biosensor based on platinum electrodes improved with electrospun TiO2 nanofibers. The resulting platinum electrode modified with electrospun TiO2 nanofiber demonstrated exceptional electrocatalysis towards H2O2 electro-oxidation. The excellent immobilization of glucose oxidase (GOx) onto the electrospun TiO2 nanofiber surfaces provides the opportunity for excellent glucose bioelectrocatalysis, which results in a decent amperometric response of 9.25 μA cm−2 mM−1 within a small response time (10 s) and a minimum detection limit of 0.01 mM. They also showed that the ZnO nanofibers improved platinum electrode offers 2.7 times more sensitivity and one order of magnitude extended detection limit compared to pristine enzymatic electrodes.
Recently, Sharma's group40 used surface modified electrospun partially aligned mesoporous anatase TiO2 nanofiber mats and fabricated an electrochemical sensing platform to detect esterified cholesterol. Fig. 14 shows a schematic representation of the fabrication of the cholesterol sensor based on electrospun TiO2 nanofibers. They controlled the electrospinning parameters to produce TiO2 nanofibers of uniform diameters ranging from 30–60 nm. The porosity of TiO2 nanofibers was organized using the sacrificial templating polymer PVP in titanium isopropoxide, which is a sol–gel precursor to TiO2. Calcination of the as spun PVP/TiO2 blend fibers removed the polymer content from the composite and generated porosity in the TiO2 fibers. The alignment of the fibers was controlled using a rotating collector at the time of electrospinning. Fig. 15a shows the unaligned nanofibers when electrospinning was completed on a static collector, whereas Fig. 15b and c demonstrate the nanofibrous mat comprised of aligned nanofibers by FESEM micrographs. The calcination was controlled to retain the pure anatase phase of the TiO2 nanofibers. Fig. 15d–f show FESEM images of the calcined TiO2 nanofibers and individual nanofibers. Oxy-plasma treatment generated several functional groups, such as –COOH and –CHO, on the TiO2 nanofibers which help in covalent bonding between the enzyme and fibers and result in a high loading of enzyme in the bioelectrode. Cholesterol esterase (ChEt)–cholesterol oxidase (ChOx) dual enzymes were immobilized by covalent interaction on the TiO2 nanofibers via EDC-NHS [N-ethyl-N0-(3-dimethylaminopropyl carbodiimide) and N-hydroxysuccinimide] chemical modification of the electrode. The resultant biosensor showed exceptional voltammetric and biocatalytic responses toward esterified cholesterol sensing with a detection limit of 0.49 mM. They claimed that the high aspect ratio, mesoporosity and high enzyme loading on the electrospun TiO2 nanofibers provide fast detection within 20 s and an exceptional sensitivity of 181.6 μA mg−1 dL−1 cm−2.
 | ||
| Fig. 14 Schematic for the biofunctionalized mesoporous TiO2 nanofiber based biosensing platform for esterified cholesterol detection. Reprinted with permission from ref. 40. Copyright (2014) American Chemical Society. | ||
 | ||
| Fig. 15 FE-SEM images of the calcined electrospun TiO2 nanofibers (a) unaligned (b) in the form of a free standing mat, and (c) magnified image of the mat. Partially aligned TiO2 nanofibers at (d) low and (e) high resolution. (f) Individual TiO2 nanofibers and in the inset: a single nanofiber. Reprinted with permission from ref. 40. Copyright (2014) American Chemical Society. | ||
Zhang et al.228 also fabricated a cell capture assay based on electrospun TiO2 nanofibers, which was applied for the detection of circulating tumor cells in colorectal and gastric cancer patients. In another study, Lee et al.70 fabricated a very efficient lab-on-a-disc biosensing device made up of an electrospun TiO2 nanofiber mat. They also reported that this device displays stable detection of serum proteins with a wide dynamic range, with merely 10 μL of whole blood in 30 min.
4.3 Electrospun Co3O4 nanofiber based interfaces for biosensing
Cobalt oxide nanostructures are extremely chemically reactive, and they have been used in various device applications such as magnetoresistive and energy storage, electronic thin films, water splitting, and chemical and photo catalysis. In addition, cobalt oxide is an eminent p-type semiconductor material, which has obtained a bigger research thrust since it is easily available, highly stabile, low costing, and a good thermos-electrical conductor. These properties help Co3O4 nanofibers to find applications in electrochemical sensing and gas sensing devices. These metal-oxide nanofibers have wonderful electrocatalytic activity towards the evolution of ozone, and oxygen. Also Co3O4 nanoparticles can be easily deposited on many support structures by electrochemical deposition and the composite fibers yield high purity cobalt oxide, which could be beneficial in many ways for biomedical and diagnostic device based applications. There are reports in the literature, including the application of electrospun Co3O4 nanofibers, for the detection of various molecules such as cysteine, glucose, propylamine, methanol and hydroquinone. In light of these findings Ramasamy et al.229 described the design of an enzyme-free highly sensitive and selective glucose biosensor using electrospun Co3O4/NiO composite nanofibers. They demonstrated that the Co3O4/NiO composite nanofibers are highly porous and homogeneously distributed in the matrix with effective inter connectivity and extended conducting channels of 160 nm diameter. The cyclic voltammetry and amperometry techniques were used to collect electrochemical sensing information on glucose and the results shows a high sensitivity of 2477 μA mM−1 cm−2 with a detection limit of 0.17 μM in a widespread linear range of 1 μM to 9.055 mM. Electrospun Co3O4 nanofibers were also used for sensitive and selective glucose detection by Ding et al.173 They suggested that electrospun Co3O4 nanofibers have extensive application potential in the field of sensor development for enzyme-free glucose detection.4.4 Electrospun MnO2 nanofibers for biosensing
MnO2 is another known inorganic metal-oxide which has been extensively explored because of its imperative claim in catalysis and electrodes in energy storage devices. In addition, manganese dioxide has also been shown to have catalytic activity in the conversion of oxygen from hydrogen peroxide. It has been shown that MnO2 nanostructures could counter with hydrogen peroxide and produce Mn2+ and O2 at the same time as swelling two hydrogen ions. There are many biosensor devices have been developed based on this benefit of H2O2 monitoring. Guo's group230 developed a microchip based on electrospun MnO2 nanofibers, which was integrated with microchannels for the controlled capture/release of cancer cells. This device was fabricated by the electrospinning of MnO2 nanofibers followed by lift-off and soft-lithography and finally, introduced into microchannels to analyze the cancerous cells in a liquid specimen. In a recent study Xiao and coworkers231 developed an efficient enzyme-free H2O2 biosensor platform using electrospun carbon nanofibers and manganese dioxide composite nanofibers.4.5 Other electrospun metal/metal-oxide nanofibers based biosensors
Nanostructured cupric oxide (CuO) has a narrow bandgap and numerous physicochemical properties.232 The electrospun nanofibrous morphology of CuO is a new striking option in various fields for example optoelectronics, catalysis, photocatalysis, and energy storage and energy conversion, including biosensing applications. Recently, an electrospun CuO nanofiber network was used to prepare a three dimensional film, which was introduced for non-enzymatic glucose sensing by Wang et al.233 Their results show that the sensor has a high sensitivity of 431.3 μAm M−1 cm−2 with a quick response (∼1 s) and long lasting stability of the working electrode. Improved sensitivity for electrospun CuO nanofiber based biosensor platforms toward nonenzymatic glucose detection sensing was studied by Liu et al.234ZrO2 is another promising metal-oxide material for use in biosensor and biomedical applications. Both in vitro and in vivo studies have established the high biocompatibility of ZrO2, particularly when it is totally decontaminated from its radioactive counterparts.235,236 Wang et al.237 applied electrospun ZrO2 nanofibers toward the analysis of phosphorylated peptides and proteins. Also, the antimicrobial and release behaviors of electrospun nisin-poly(vinyl alcohol)/wheat gluten/ZrO2 nanofibrous membranes was reported recently by Wang et al.238
Yuan et al.239 reported a nonenzymatic electrochemical glucose sensor fabricated by graphene oxide and an NiO modified glassy carbon electrode. The fabricated device can detect glucose in the concentration range of 3.13 μM to 3.05 mM with a detection limit of 1 μM.
For nonenzymatic glucose and hydrogen peroxide sensing applications, perovskite LaNiO3 metal-oxide nanofibers have been fabricated efficaciously by the electrospinning technique and consecutive calcinations. The improved electrode presented extraordinary electrocatalysis towards the oxidation of glucose and H2O2. The H2O2 sensor demonstrated a small detection limit of 33.9 nM with a wide-ranging linear detection boundary of 0.05–1000 mM.240
In addition to metal-oxide nanofibers, there are several reports on electrochemical biosensors based on polymer and carbon nanofibers decorated with metal, metal-oxides, graphene, carbon nanotubes and other functional nanomaterials. For example, recently a new biosensor was constructed by electrospinning gold with immobilized fructose dehydrogenase enzyme.241 The gold fibers were prepared by electrospinning a blend of poly(acrylonitrile)/HAuCl4. An enzyme, namely, fructose dehydrogenase was immobilized on the gold nanofiber surface by covalent interaction through glutaraldehyde (a crosslinking agent) to a thin layer of cystamine. The KM of the immobilized enzyme was 5 mM which further indicates good immobilization on the gold surface. The resultant sensor senses fructose very quickly (<2.2 s) and is stable up to 20 operating cycles with a detection limit of 11.7 μM.
Carbon nanofibers have also been used in biosensing applications because of their good electrical properties, which are comparable to carbon nanotubes, and high mechanical strength. Besides, carbon nanofibers have superior surface-active groups as compared to the glassy surface of carbon nanotubes, which make them a potential candidate that can afford a huge enzyme loading and can be imparted as transducing materials for sensing.242,243 In this interest, Tang et al.,244 developed three electrochemical sensing platforms using carbon nanofibers, which can be used for the straightforward measurable detection of L-cysteine, L-tryptophan, and L-tyrosine amino acid molecules. These sensors have a detection limit of 0.1 μM and the stable detection range of 0.1–119, 0.2–107 and 0.15–64 μM, respectively, for the three targeted analytes. Also very recently a hydroquinone sensor was fabricated by Li et al.,245 in which electrospun carbon nanofibers were decorated with NiCu alloy nanoparticles by high temperature carbonization. The sensing platform achieved a sensitivity of 1.5 μA μM−1 with a 90 nM detection limit in the range of 0.4–2.37 μM hydroquinone concentration.
Various other electrospun metal-oxide nanofibers, including metal, carbon or even other metal oxide nanofibers, have been employed for the fabrication of electrochemical biosensing devices toward an extensive choice of bioanalytes. A summary of the electrochemical biosensors based on electrospun metal-oxide nanofibers is given in Table 1.
| Nanofibrous electrode | Fiber diameter (nm) | Analyte | Linear range (μM) | Sensitivity | Detection limit (μM) | Ref. |
|---|---|---|---|---|---|---|
| ZnO/GOx | 100 | Glucose | — | 69 μA mM−1 cm−2 | 10 | Huang et al.251 |
| TiO2/Pt | 72.6 | Hydrazine | −1030 | 44.42 μA mM−1 cm−2 | 0.142 | Ding et al.252 |
| TiO2/graphene oxide/carbon | 70 | Adenine | 0.1–10 | 0.1823 μA μM−1 | 1.71 | Arvand et al.253 |
| TiO2/graphite oxide | 40–70 | Levodopa | 0.3–60 | 0.0806 μA μM−1 | 15.94 | Arvand et al.254 |
| CuO/Pd | 90–140 | Glucose | 0.2–2500 | 1061.4 μA mM−1 cm−2 | 0.019 | Wang et al.255 |
| SiO2/Au | — | H2O2 | 5–1000 | — | 2 | Shen et al.256 |
| La0.88Sr0.12MnO3/carbon | 300–400 | Glucose | 0.05–100 | 1111.11 μA mM−1 cm−2 | 0.0312 | Xu et al.257 |
| Ag/CeO2–Au/carbon | 200 | Levofloxacin | 0.03–10 | 1240 μA mM−1 cm−2 | 0.01 | Tang et al.258 |
| Fe3O4 | 200 | Aflatoxin B1 | 0.000159–0.636 | — | 0.00636 | Xu et al.259 |
| CoFe2O4 | 200–300 | Hydrazine | 100–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
503 μA mM−1 cm−2 | 1000 | Liu et al.260 |
| RuO2–TiO2 | 100 | Ascorbic acid | 10–1500 | 268.2 ± 3.7 μA mM−1 cm−2 | 1.8 | Kim et al.261 |
| Carbon doped TiO2/graphene foam | 72 | Breast cancer biomarkers (ErbB2) | 0.000001–0.1 | 123.5 kΩ pM−1 | 0.000001 | Ali et al.262 |
| MnCo2O4/graphene | 150–300 | Glucose | 0.005–800 | 1813.8 μA mM−1 cm−2 | 0.001 | Zhang et al.263 |
Moreover, thin films are frequently employed for sensor fabrication to increase the performance of the sensor. This has been well explored, in that the sensitivity of a film based sensor is proportionate to its surface area per unit mass. Interestingly, thin films formed by nanofibers have surface areas that are several orders of magnitude greater than films and thus their sensitivities are also higher. Kumar's group recently reported that the sensitivity of their fabricated sensors was enhanced by assembling their components in an electrospun nanofibrous membrane.246 Additionally, when metal-oxides are incorporated into nanofibers, the sensitivities of the sensors increase further. A large available surface with decorated nanoparticles is the advantage that metal-oxide nanofibers have over polymer nanofiber based sensor substrates. Nanofibrous supports with suitable metal-oxide nanostructure architectures can provide a unique environment for biosensing due to better chemical stability, enhanced conductivity, excellent electrocatalytic activity and ability to facilitate direct electron transfer.
Interestingly, the presence of a certain oxide is beneficial for specific sensor devices owing to its improved interaction with targeted analytes and better immobilization of biomolecules on nanostructured surfaces.247 This is the key requirement for the nanofabrication of biosensors with high sensitivity and specificity. Whitesides's group demonstrated that the headgroups of organic molecules can preferentially bind to specific metals, metal-oxides, and semiconductors.248 For example, metal-oxide surfaces, such as silicon dioxide, with OH− groups can respond to alkyltrichlorosilanes or alkyltriethoxysilanes, such as APTES (3-aminopropyltriethoxysilane), and create covalent siloxane bonds at the surface of the oxides.249,250
5. Conclusions and remarks
There are countless progressions in the field of biosensors and laterally many facades in the past decades. Biosensors and bioelectronics have established intensified importance over the past decade, ever since they have been shown to be capable candidates for lower detection limits with fast analysis at reasonably low prices. Technological improvements have delivered the apparatus and materials required to build a biochip which can be combined with microfluidic arrangements, analysis probes, digital electronics, samplers, and logic circuitry. These biosensor platforms are also encouraging applicants for label free, miniaturized, reagent free, and low cost real time point-of-care application. In view of their biomedical use, this cost benefit would permit the growth and expansion of exceptionally low cost, reusable biochips that can be earmarked for use in the home medical diagnostics of diseases and infections, which are deprived of the time intensive inevitability of sample transfer for analysis.Electrospun metal-oxide nanofibers have exhibited huge advantages for applications in biosensing biomedical and lab on chip devices because of the handy, effectual, and cost effective approach for the fabrication of these nanostructures from a rich selection of materials. As for their application in biosensors, electrospun metal-oxide nanofibers have exhibited high sensitivity for sensing because of their efficient charge separation and transportation and high enzyme loading capability by superior adsorption, entrapment and strong covalent bonding with biomolecules, which are mainly ascribed to their high aspect ratio, enormous accessible specific surface areas and vast interconnected porosity. After carefully reviewing the latest representative publications in the field of electrochemical biosensors, the purpose of various electrospun metal-oxide nanofibers towards both the enzymatic and enzyme-free detection of different biomolecules have been explored in this review. Electrospun ZnO, TiO2, Co3O4 and MnO2 nanofibers have been acclaimed to be the most efficient metal-oxides for the biosensor fabrication. The inquiries also recommend that the coexistence of metal-oxides with different allotropes of nanophase carbon and metal nanoparticles is advantageous for enzyme immobilization and biocatalysis during the electrochemical sensing of biomolecules. It is also notable that the surface functionalization of nanofibers by chemical and/or physical modification further generates covalent functionalities which favor efficient enzyme immobilization and electrocatalysis. Also, in terms of their use in electrochemical biosensing, working electrodes fabricated by electrospun nanofibers have established high specific capacitance, attention-grabbing low detection limits and improved cycling operation stability owing to their unique fibrous morphology and good crystallinity in the nanophase, including large surface area to volume ratio and nanoscopic diameter. In spite of their benefits, the use of electrospun metal-oxide nanofibers for biosensing devices also faces a few challenges such as, in enzyme-based biosensing, the efficient immobilization of enzymes onto the substrate through the fiber network, and the adhesion between substrate and fibers is an issue that needs to be addressed well. Concerning this, the development of electrobiocatalysts that are competent and robust, meanwhile retaining redox reactivity and stability, will be a significant task in future research. In light of this concern, the engineering of electrospun metal-oxide/organometallic perovskite nanofibers or composite metal-oxide/organometallic perovskite/graphene composite nanofibers with diverse hierarchical morphologies as electrocatalysts may be a viable and favorable solution.
Conflict of interest
There is no conflict of interest about this article.References
- N. Orgovan, D. Patko, C. Hos, S. Kurunczi, B. Szabó, J. J. Ramsden and R. Horvath, Adv. Colloid Interface Sci., 2014, 211, 1–16 CrossRef CAS PubMed.
- J. A. Goode, J. V. H. Rushworth and P. A. Millner, Langmuir, 2014, 31, 6267–6276 CrossRef PubMed.
- L. Su, W. Jia, C. Hou and Y. Lei, Biosens. Bioelectron., 2011, 26, 1788–1799 CrossRef CAS PubMed.
- A. Biswas, I. S. Bayer, A. S. Biris, T. Wang, E. Dervishi and F. Faupel, Adv. Colloid Interface Sci., 2012, 170, 2–27 CrossRef CAS PubMed.
- J. H. Wendorff, S. Agarwal and A. Greiner, Electrospinning: Materials, Processing, and Applications, Wiley, 2012 Search PubMed.
- S. S. Mali, P. S. Patil and C. K. Hong, ACS Appl. Mater. Interfaces, 2014, 6, 1688–1696 CAS.
- D. Nan, J.-G. Wang, Z.-H. Huang, L. Wang, W. Shen and F. Kang, Electrochem. Commun., 2013, 34, 52–55 CrossRef CAS.
- Q. L. Loh and C. Choong, Tissue Eng., Part B, 2013, 19, 485–502 CrossRef CAS PubMed.
- T. Wang and S. Kumar, Journal of Applied Polymer Science, 2006, 102, 1023–1029 CrossRef CAS.
- H.-S. Bae, A. Haider, K. M. K. Selim, D.-Y. Kang, E.-J. Kim and I.-K. Kang, J. Polym. Res., 2013, 20, 1–7 CAS.
- K. Mondal, M. A. Ali, S. Srivastava, B. D. Malhotra and A. Sharma, Sens. Actuators, B, 2016, 229, 82–91 CrossRef CAS.
- Y. Zhang and G. C. Rutledge, Macromolecules, 2012, 45, 4238–4246 CrossRef CAS.
- N. Bhattarai, D. Edmondson, O. Veiseh, F. A. Matsen and M. Zhang, Biomaterials, 2005, 26, 6176–6184 CrossRef CAS PubMed.
- A. Frenot, M. W. Henriksson and P. Walkenström, J. Appl. Polym. Sci., 2007, 103, 1473–1482 CrossRef CAS.
- D. Li, Z. Pang, X. Chen, L. Luo, Y. Cai and Q. Wei, Beilstein J. Nanotechnol., 2014, 5, 346–354 CrossRef PubMed.
- J.-i. Hahm, Sensors, 2011, 11, 3327–3355 CrossRef CAS PubMed.
- G. Ren, X. Xu, Q. Liu, J. Cheng, X. Yuan, L. Wu and Y. Wan, React. Funct. Polym., 2006, 66, 1559–1564 CrossRef CAS.
- J. Y. Lee, C. A. Bashur, A. S. Goldstein and C. E. Schmidt, Biomaterials, 2009, 30, 4325–4335 CrossRef CAS PubMed.
- D. Li, M. W. Frey and A. J. Baeumner, J. Membr. Sci., 2006, 279, 354–363 CrossRef CAS.
- H. S. Gibson, P. Gibson, P. Tsai, P. Gupta and G. Wilkes, INJ Winter, 2004, 13, 39–46 Search PubMed.
- T. Lin, H. Wang and X. Wang, Adv. Mater., 2005, 17, 2699–2703 CrossRef CAS.
- J. Wang and Y. Lin, TrAC, Trends Anal. Chem., 2008, 27, 619–626 CrossRef CAS PubMed.
- R. M. Anwar, K. Vattipalli, E. Myrah, R. Asmatulu and S. Prasad, Adv. Sci., Eng. Med., 2013, 5, 633–640 CrossRef.
- K. Chatterjee, S. Sarkar, K. Jagajjanani Rao and S. Paria, Adv. Colloid Interface Sci., 2014, 209, 8–39 CrossRef CAS PubMed.
- M. Sireesha, V. Jagadeesh Babu and S. Ramakrishna, RSC Adv., 2015, 5, 103308–103314 RSC.
- F. Croisier and C. Jérôme, Eur. Polym. J., 2013, 49, 780–792 CrossRef CAS.
- P. A. Palod and V. Singh, Mater. Sci. Eng., C, 2015, 55, 420–430 CrossRef CAS PubMed.
- U. Saxena and A. B. Das, Biosens. Bioelectron., 2016, 75, 196–205 CrossRef CAS PubMed.
- C. Dhand, P. R. Solanki, M. Datta and B. D. Malhotra, Electroanalysis, 2010, 22, 2683–2693 CrossRef CAS.
- W. Yan, X. Chen, X. Li, X. Feng and J.-J. Zhu, J. Phys. Chem. B, 2008, 112, 1275–1281 CrossRef CAS PubMed.
- M. A. Ali, C. Singh, K. Mondal, S. Srivastava, A. Sharma and B. D. Malhotra, ACS Appl. Mater. Interfaces, 2016, 7646–7656 CAS.
- M. Bhambi, G. Sumana, B. D. Malhotra and C. S. Pundir, Artif. Cells, Blood Substitutes, Biotechnol., 2010, 38, 178–185 CrossRef CAS PubMed.
- E. Ghasemi, H. Ziyadi, A. M. Afshar and M. Sillanpää, Chem. Eng. J., 2015, 264, 146–151 CrossRef CAS.
- J. Cai, H. Niu, Z. Li, Y. Du, P. Cizek, Z. Xie, H. Xiong and T. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 14946–14953 CAS.
- B. M. Shilpa, B. M. Basavaraja, S. B. Majumder and A. Sharma, J. Mater. Chem. A, 2015, 3, 5344–5351 CAS.
- J.-Y. Chen, C.-C. Kuo, C.-S. Lai, W.-C. Chen and H.-L. Chen, Macromolecules, 2011, 44, 2883–2892 CrossRef CAS.
- B. Mordina, R. K. Tiwari, D. K. Setua and A. Sharma, RSC Adv., 2015, 5, 19091–19105 RSC.
- P. Singh, K. Mondal and A. Sharma, J. Colloid Interface Sci., 2013, 394, 208–215 CrossRef CAS PubMed.
- T.-A. Nguyen, S. Park, J. B. Kim, T. K. Kim, G. H. Seong, J. Choo and Y. S. Kim, Sens. Actuators, B, 2011, 160, 549–554 CrossRef CAS.
- K. Mondal, M. A. Ali, V. V. Agrawal, B. D. Malhotra and A. Sharma, ACS Appl. Mater. Interfaces, 2014, 6, 2516–2527 CAS.
- T. Amna, M. S. Hassan, F. Sheikh, H. Lee, K.-S. Seo, D. Yoon and I. H. Hwang, Appl. Microbiol. Biotechnol., 2013, 97, 1725–1734 CrossRef CAS PubMed.
- A. A. Madhavan, A. Mohandas, A. Licciulli, K. P. Sanosh, P. Praveen, R. Jayakumar, S. V. Nair, A. S. Nair and A. Balakrishnan, RSC Adv., 2013, 3, 25312–25316 RSC.
- A. F. Lotus, E. T. Bender, E. A. Evans, R. D. Ramsier, D. H. Reneker and G. G. Chase, J. Appl. Phys., 2008, 103, 024910 CrossRef.
- Y. Shen, S. Turner, P. Yang, G. Van Tendeloo, O. I. Lebedev and T. Wu, Nano Lett., 2014, 14, 4342–4351 CrossRef CAS PubMed.
- H. K. Yu and J.-L. Lee, Sci. Rep., 2014, 4, 6589 CrossRef CAS PubMed.
- S. Yoo, S. A. Akbar and K. H. Sandhage, Ceram. Int., 2004, 30, 1121–1126 CrossRef CAS.
- H. Wu, W. Pan, D. Lin and H. Li, J. Adv. Ceram., 2012, 1, 2–23 CrossRef CAS.
- Y. Dai, W. Liu, E. Formo, Y. Sun and Y. Xia, Polym. Adv. Technol., 2010, 22, 326–338 CrossRef.
- P. K. Panda, Trans. Indian Ceram. Soc., 2007, 66, 65–76 CrossRef CAS.
- D. Li and Y. Xia, Nano Lett., 2004, 4, 933–938 CrossRef CAS.
- Z.-M. Huang, Y. Z. Zhang, M. Kotaki and S. Ramakrishna, Compos. Sci. Technol., 2003, 63, 2223–2253 CrossRef CAS.
- A. L. Yarin, Polym. Adv. Technol., 2011, 22, 310–317 CrossRef CAS.
- K. Nayani, H. Katepalli, C. S. Sharma, A. Sharma, S. Patil and R. Venkataraghavan, Ind. Eng. Chem. Res., 2012, 51, 1761–1766 CrossRef CAS.
- X. Zhang, D. Liu, L. Li and T. You, Sci. Rep., 2015, 5, 9885 CrossRef CAS PubMed.
- J. E. Panels and Y. L. Joo, J. Nanomater., 2006, 2006, 10 Search PubMed.
- T. Senthil and S. Anandhan, J. Colloid Interface Sci., 2014, 432, 285–296 CrossRef CAS PubMed.
- F. R. Lamastra, F. Nanni, L. Camilli, R. Matassa, M. Carbone and G. Gusmano, Chem. Eng. J., 2010, 162, 430–435 CrossRef CAS.
- C.-L. Zhang and S.-H. Yu, Chem. Soc. Rev., 2014, 43, 4423–4448 RSC.
- J. Njuguna, Structural Nanocomposites: Perspectives for Future Applications, Springer, 2013 Search PubMed.
- Y. Okawa, N. Yokoyama, Y. Sakai and F. Shiba, Anal. Chem. Res., 2015, 5, 1–8 CrossRef CAS.
- R. S. Freire, C. A. Pessoa, L. D. Mello and L. T. Kubota, J. Braz. Chem. Soc., 2003, 14, 230–243 CrossRef CAS.
- A. Ghindilis, Biochem. Soc. Trans., 2000, 28, 84–89 CrossRef CAS PubMed.
- S. Huang, Y. Ding, Y. Liu, L. Su, R. Filosa and Y. Lei, Electroanalysis, 2011, 23, 1912–1920 CrossRef CAS.
- D. Liu, Q. Guo, X. Zhang, H. Hou and T. You, J. Colloid Interface Sci., 2015, 450, 168–173 CrossRef CAS PubMed.
- C. Huang, S. J. Soenen, J. Rejman, J. Trekker, L. Chengxun, L. Lagae, W. Ceelen, C. Wilhelm, J. Demeester and S. C. De Smedt, Adv. Funct. Mater., 2012, 22, 2479–2486 CrossRef CAS.
- A. Townsend-Nicholson and S. N. Jayasinghe, Biomacromolecules, 2006, 7, 3364–3369 CrossRef CAS PubMed.
- A. Babakhanian, T. Momeneh, P. Aberoomand-azar, S. Kaki, M. Torki, S. Hossein Kiaie, E. Sadeghi and F. Dabirian, Analyst, 2015, 140, 7761–7767 RSC.
- T. Yang, N. Zhou, Y. Zhang, W. Zhang, K. Jiao and G. Li, Biosens. Bioelectron., 2009, 24, 2165–2170 CrossRef CAS PubMed.
- M. A. Ali, K. Mondal, C. Singh, B. Dhar Malhotra and A. Sharma, Nanoscale, 2015, 7, 7234–7245 RSC.
- W. S. Lee, V. Sunkara, J.-R. Han, Y.-S. Park and Y.-K. Cho, Lab Chip, 2015, 15, 478–485 RSC.
- H. Bagheri, Z. Ayazi, A. Aghakhani and N. Alipour, J. Sep. Sci., 2012, 35, 114–120 CrossRef CAS PubMed.
- J. S. Lee, O. S. Kwon, S. J. Park, E. Y. Park, S. A. You, H. Yoon and J. Jang, ACS Nano, 2011, 5, 7992–8001 CrossRef CAS PubMed.
- D. Qi, X. Kang, L. Chen, Y. Zhang, H. Wei and Z. Gu, Anal. Bioanal. Chem., 2008, 390, 929–938 CrossRef CAS PubMed.
- J. E. Oliveira, V. P. Scagion, V. Grassi, D. S. Correa and L. H. C. Mattoso, Sens. Actuators, B, 2012, 171–172, 249–255 CrossRef CAS.
- W. E. Teo and S. Ramakrishna, Nanotechnology, 2006, 17, R89 CrossRef CAS PubMed.
- L. Persano, A. Camposeo, C. Tekmen and D. Pisignano, Macromol. Mater. Eng., 2013, 298, 504–520 CrossRef CAS.
- S. Chigome and N. Torto, Anal. Chim. Acta, 2011, 706, 25–36 CrossRef CAS PubMed.
- H. S. Yoo, T. G. Kim and T. G. Park, Adv. Drug Delivery Rev., 2009, 61, 1033–1042 CrossRef CAS PubMed.
- Y. C. Ahn, S. K. Park, G. T. Kim, Y. J. Hwang, C. G. Lee, H. S. Shin and J. K. Lee, Curr. Appl. Phys., 2006, 6, 1030–1035 CrossRef.
- J. Huang, S. Virji, B. H. Weiller and R. B. Kaner, J. Am. Chem. Soc., 2003, 125, 314–315 CrossRef CAS PubMed.
- D. Aussawasathien, J. H. Dong and L. Dai, Synth. Met., 2005, 154, 37–40 CrossRef CAS.
- A. G. MacDiarmid, W. E. Jones, I. D. Norris, J. Gao, A. T. Johnson, N. J. Pinto, J. Hone, B. Han, F. K. Ko and H. Okuzaki, Synth. Met., 2001, 119, 27–30 CrossRef CAS.
- J. Pu, X. Yan, Y. Jiang, C. Chang and L. Lin, Sens. Actuators, A, 2010, 164, 131–136 CrossRef CAS.
- L. Ji, Z. Lin, A. J. Medford and X. Zhang, Carbon, 2009, 47, 3346–3354 CrossRef CAS.
- Q. P. Pham, U. Sharma and A. G. Mikos, Biomacromolecules, 2006, 7, 2796–2805 CrossRef CAS PubMed.
- T. Krishnamoorthy, M. Z. Tang, A. Verma, A. S. Nair, D. Pliszka, S. G. Mhaisalkar and S. Ramakrishna, J. Mater. Chem., 2012, 22, 2166–2172 RSC.
- V. P. Secasanu, C. K. Giardina and Y. Wang, Biotechnol. Prog., 2009, 25, 1169–1175 CrossRef CAS PubMed.
- T. Maitra, S. Sharma, A. Srivastava, Y.-K. Cho, M. Madou and A. Sharma, Carbon, 2012, 50, 1753–1761 CrossRef CAS.
- D. Li, Y. Wang and Y. Xia, Nano Lett., 2003, 3, 1167–1171 CrossRef CAS.
- W. E. Teo and S. Ramakrishna, Nanotechnology, 2005, 16, 1878 CrossRef CAS.
- M. R. Badrossamay, H. A. McIlwee, J. A. Goss and K. K. Parker, Nano Lett., 2010, 10, 2257–2261 CrossRef CAS PubMed.
- V. Beachley and X. Wen, Mater. Sci. Eng., C, 2009, 29, 663–668 CrossRef CAS PubMed.
- C. J. Thompson, G. G. Chase, A. L. Yarin and D. H. Reneker, Polymer, 2007, 48, 6913–6922 CrossRef CAS.
- K. Mondal, S. Bhattacharyya and A. Sharma, Ind. Eng. Chem. Res., 2014, 53, 18900–18909 CrossRef CAS.
- H. Shao, J. Fang, H. Wang and T. Lin, RSC Adv., 2015, 5, 14345–14350 RSC.
- S. Fukushima, Y. Karube and H. Kawakami, Polym. J., 2010, 42, 514–518 CrossRef CAS.
- D. Fallahi, M. Rafizadeh, N. Mohammadi and B. Vahidi, e-Polymers, 2009, 9, 1250 Search PubMed.
- S. Zargham, S. Bazgir, A. Tavakoli, A. S. Rashidi and R. Damerchely, J. Eng. Fibers Fabr., 2012, 7, 42–49 CAS.
- Y. Liu, L. Dong, J. Fan, R. Wang and J.-Y. Yu, J. Appl. Polym. Sci., 2010, 120, 592–598 CrossRef.
- A. G. Sener, A. S. Altay and F. Altay, Electrical and Electronics Engineering (ELECO), 7th International Conference on IEEE, 2011, p. I-324 Search PubMed.
- K. Garg and G. L. Bowlin, Biomicrofluidics, 2011, 5, 013403 CrossRef PubMed.
- H. R. Darrell and C. Iksoo, Nanotechnology, 1996, 7, 216 CrossRef.
- X. Yuan, Y. Zhang, C. Dong and J. Sheng, Polym. Int., 2004, 53, 1704–1710 CrossRef CAS.
- C. Mit-uppatham, M. Nithitanakul and P. Supaphol, Macromol. Chem. Phys., 2004, 205, 2327–2338 CrossRef CAS.
- C. L. Casper, J. S. Stephens, N. G. Tassi, D. B. Chase and J. F. Rabolt, Macromolecules, 2004, 37, 573–578 CrossRef CAS.
- R. Khajavi and M. Abbasipour, Sci. Iran., 2012, 19, 2029–2034 CrossRef CAS.
- D. Li and Y. Xia, Adv. Mater., 2004, 16, 1151–1170 CrossRef CAS.
- H. Hou, Z. Jun, A. Reuning, A. Schaper, J. H. Wendorff and A. Greiner, Macromolecules, 2002, 35, 2429–2431 CrossRef CAS.
- M. Wei, J. Lee, B. Kang and J. Mead, Macromol. Rapid Commun., 2005, 26, 1127–1132 CrossRef CAS.
- P. D. Dalton, D. Klee and M. Moiller, Polymer, 2005, 46, 611–614 CrossRef CAS.
- A. Greiner and J. H. Wendorff, Angew. Chem., Int. Ed., 2007, 46, 5670–5703 CrossRef CAS PubMed.
- K. Mondal and A. Sharma, RSC Adv., 2016, 6, 83589–83612 RSC.
- S. Agarwal, J. H. Wendorff and A. Greiner, Polymer, 2008, 49, 5603–5621 CrossRef CAS.
- J. Sundaramurthy, N. Li, P. S. Kumar and S. Ramakrishna, Biofuel Res. J., 2014, 1, 44–54 CrossRef CAS.
- S.-H. Park, H.-R. Jung and W.-J. Lee, Electrochim. Acta, 2013, 102, 423–428 CrossRef CAS.
- D. Crespy, K. Friedemann and A.-M. Popa, Macromol. Rapid Commun., 2012, 33, 1978–1995 CrossRef CAS PubMed.
- N. Horzum, R. Muñoz-Espí, G. Glasser, M. M. Demir, K. Landfester and D. Crespy, ACS Appl. Mater. Interfaces, 2012, 4, 6338–6345 CAS.
- T. Yao, S. Ramakrishna and S. Peng, in Zero-Carbon Energy Kyoto 2011, Springer, Japan, 2011, pp. 97–108 Search PubMed.
- X. Shi, W. Zhou, D. Ma, Q. Ma, D. Bridges, Y. Ma and A. Hu, J. Nanomater., 2015, 2015, 20 Search PubMed.
- L. Li, W. H. Meyer, G. Wegner and M. Wohlfahrt-Mehrens, Adv. Mater., 2005, 17, 984–988 CrossRef CAS.
- G. Lu, I. Lieberwirth and G. Wegner, J. Am. Chem. Soc., 2006, 128, 15445–15450 CrossRef CAS PubMed.
- S.-S. Choi, S. Lee, S. Im, S. Kim and Y. Joo, J. Mater. Sci. Lett., 2003, 22, 891–893 CrossRef CAS.
- Z. Ma, H. Ji, Y. Teng, G. Dong, J. Zhou, D. Tan and J. Qiu, J. Colloid Interface Sci., 2011, 358, 547–553 CrossRef CAS PubMed.
- W. Shi, W. Lu and L. Jiang, J. Colloid Interface Sci., 2009, 340, 291–297 CrossRef CAS PubMed.
- Y.-z. Chen, Z.-p. Zhang, J. Yu and Z.-X. Guo, J. Polym. Sci., Part B: Polym. Phys., 2009, 47, 1211–1218 CrossRef CAS.
- K. Friedemann, T. Corrales, M. Kappl, K. Landfester and D. Crespy, Small, 2012, 8, 144–153 CrossRef CAS PubMed.
- K. Masaki, D. Bin and S. Seimei, Nanotechnology, 2007, 18, 315602 CrossRef.
- J.-M. Lim, J. H. Moon, G.-R. Yi, C.-J. Heo and S.-M. Yang, Langmuir, 2006, 22, 3445–3449 CrossRef CAS PubMed.
- X.-C. Zhang, Y.-Z. Chen, J. Yu and Z.-X. Guo, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1683–1689 CrossRef CAS.
- N. Horzum, D. Taşçıoglu, S. Okur and M. M. Demir, Talanta, 2011, 85, 1105–1111 CrossRef CAS PubMed.
- C. Wessel, R. Ostermann, R. Dersch and B. M. Smarsly, J. Phys. Chem. C, 2013, 115, 362–372 Search PubMed.
- H. Wu and W. Pan, J. Am. Ceram. Soc., 2006, 89, 699–701 CrossRef CAS.
- M. Kanjwal, N. M. Barakat, F. Sheikh, S. Park and H. Kim, Macromol. Res., 2010, 18, 233–240 CrossRef CAS.
- D. Lin, H. Wu, R. Zhang and W. Pan, Chem. Mater., 2009, 21, 3479–3484 CrossRef CAS.
- H. Wu, L. Hu, M. W. Rowell, D. Kong, J. J. Cha, J. R. McDonough, J. Zhu, Y. Yang, M. D. McGehee and Y. Cui, Nano Lett., 2010, 10, 4242–4248 CrossRef CAS PubMed.
- N. A. M. Barakat, B. Kim and H. Y. Kim, J. Phys. Chem. C, 2009, 113, 531–536 CAS.
- X. Fang and D. H. Reneker, J. Macromol. Sci., Part B: Phys., 1997, 36, 169–173 CrossRef.
- J. D. Schiffman and C. L. Schauer, Polym. Rev., 2008, 48, 317–352 CrossRef CAS.
- J. A. Lee, K. C. Krogman, M. Ma, R. M. Hill, P. T. Hammond and G. C. Rutledge, Adv. Mater., 2009, 21, 1252–1256 CrossRef CAS.
- J. S. Tobin, A. J. Turinske, N. Stojilovic, A. F. Lotus and G. G. Chase, Curr. Appl. Phys., 2012, 12, 919–923 CrossRef.
- M. Nasr, A. Abou Chaaya, N. Abboud, M. Bechelany, R. Viter, C. Eid, A. Khoury and P. Miele, Superlattices Microstruct., 2015, 77, 18–24 CrossRef CAS.
- A. Kumar, R. Jose, K. Fujihara, J. Wang and S. Ramakrishna, Chem. Mater., 2007, 19, 6536–6542 CrossRef CAS.
- B. R. Eggins, Chemical sensors and biosensors, John Wiley & Sons, 2008 Search PubMed.
- N. J. Ronkainen, H. B. Halsall and W. R. Heineman, Chem. Soc. Rev., 2010, 39, 1747–1763 RSC.
- P. D'Orazio, Clin. Chim. Acta, 2003, 334, 41–69 CrossRef.
- M. J. Schoning and A. Poghossian, Analyst, 2002, 127, 1137–1151 RSC.
- D. Grieshaber, R. MacKenzie, J. Voros and E. Reimhult, Sensors, 2008, 8, 1400 CrossRef CAS.
- A. Chaubey and B. D. Malhotra, Biosens. Bioelectron., 2002, 17, 441–456 CrossRef CAS PubMed.
- V. M. Mirsky, M. Riepl and O. S. Wolfbeis, Biosens. Bioelectron., 1997, 12, 977–989 CrossRef CAS PubMed.
- C. Wittmann, A. Guiseppi-Elie and L. Lingerfelt, in Immobilisation of DNA on Chips I, Springer, Berlin Heidelberg, 2005, vol. 260, pp. 161–186 Search PubMed.
- D. R. Thévenot, K. Toth, R. A. Durst and G. S. Wilson, Biosens. Bioelectron., 2001, 16, 121–131 CrossRef.
- M. Ben Ali, Y. Korpan, M. Gonchar, A. El'skaya, M. A. Maaref, N. Jaffrezic-Renault and C. Martelet, Biosens. Bioelectron., 2006, 22, 575–581 CrossRef CAS PubMed.
- Y. m. Bon Saint Côme, H. l. n. Lalo, Z. Wang, M. Etienne, J. Gajdzik, G.-W. Kohring, A. Walcarius, R. Hempelmann and A. Kuhn, Langmuir, 2011, 27, 12737–12744 CrossRef PubMed.
- P. R. Solanki, A. Kaushik, V. V. Agrawal and B. D. Malhotra, NPG Asia Mater., 2011, 3, 17–24 CrossRef.
- M. Mehrvar and M. Abdi, Anal. Sci., 2004, 20, 1113–1126 CrossRef CAS PubMed.
- A. T. Sage, J. D. Besant, B. Lam, E. H. Sargent and S. O. Kelley, Acc. Chem. Res., 2014, 47, 2417–2425 CrossRef CAS PubMed.
- Y. Shao, J. Wang, H. Wu, J. Liu, I. A. Aksay and Y. Lin, Electroanalysis, 2010, 22, 1027–1036 CrossRef CAS.
- A. Poghossian and M. J. Schöning, Electroanalysis, 2014, 26, 1197–1213 CrossRef CAS.
- C. Zhu, G. Yang, H. Li, D. Du and Y. Lin, Anal. Chem., 2014, 87, 230–249 CrossRef PubMed.
- M.-J. Song, S. W. Hwang and D. Whang, Talanta, 2010, 80, 1648–1652 CrossRef CAS PubMed.
- Y.-S. Hsieh, B.-D. Hong and C.-L. Lee, Microchim. Acta, 2015, 1–6 Search PubMed.
- J. Wang, D. F. Thomas and A. Chen, Anal. Chem., 2008, 80, 997–1004 CrossRef CAS PubMed.
- Y. Li, X. Zhai, H. Wang, X. Liu, L. Guo, X. Ji, L. Wang, H. Qiu and X. Liu, Microchim. Acta, 2015, 182, 1877–1884 CrossRef CAS.
- Z.-D. Gao, Y. Han, Y. Wang, J. Xu and Y.-Y. Song, Sci. Rep., 2013, 3, 3323 Search PubMed.
- M. M. Khan, S. A. Ansari, J. Lee and M. H. Cho, Mater. Sci. Eng., C, 2013, 33, 4692–4699 CrossRef CAS PubMed.
- L. Li, Z. Du, S. Liu, Q. Hao, Y. Wang, Q. Li and T. Wang, Talanta, 2010, 82, 1637–1641 CrossRef CAS PubMed.
- S. Palanisamy, S.-M. Chen and R. Sarawathi, Sens. Actuators, B, 2012, 166–167, 372–377 CrossRef CAS.
- J. Song, L. Xu, C. Zhou, R. Xing, Q. Dai, D. Liu and H. Song, ACS Appl. Mater. Interfaces, 2013, 5, 12928–12934 CAS.
- J. N. Tiwari, V. Vij, K. C. Kemp and K. S. Kim, ACS Nano, 2015, 46–80 Search PubMed.
- Z. Wang, Y. Hu, W. Yang, M. Zhou and X. Hu, Sensors, 2012, 12, 4860–4869 CrossRef CAS PubMed.
- J. Zhu, J. Jiang, J. Liu, R. Ding, Y. Li, H. Ding, Y. Feng, G. Wei and X. Huang, RSC Adv., 2011, 1, 1020–1025 RSC.
- C. Zhou, L. Xu, J. Song, R. Xing, S. Xu, D. Liu and H. Song, Sci. Rep., 2014, 4, 7382 CrossRef CAS PubMed.
- Y. Ding, Y. Wang, L. Su, M. Bellagamba, H. Zhang and Y. Lei, Biosens. Bioelectron., 2010, 26, 542–548 CrossRef CAS PubMed.
- M. M. Rahman, A. J. Saleh Ahammad, J.-H. Jin, S. J. Ahn and J.-J. Lee, Sensors, 2010, 10, 4855–4886 CrossRef CAS PubMed.
- X. Chen, G. Wu, Z. Cai, M. Oyama and X. Chen, Microchim. Acta, 2014, 181, 689–705 CrossRef CAS.
- Y. Miao, L. Ouyang, S. Zhou, L. Xu, Z. Yang, M. Xiao and R. Ouyang, Biosens. Bioelectron., 2014, 53, 428–439 CrossRef CAS PubMed.
- S. Cynthia and D. M. Shelley, Recent Pat. Eng., 2008, 2, 195–200 CrossRef.
- S. Datta, L. R. Christena and Y. R. S. Rajaram, 3 Biotech, 2012, 3, 1–9 CrossRef.
- S. Sakai, Y. Liu, T. Yamaguchi, R. Watanabe, M. Kawabe and K. Kawakami, Biotechnol. Lett., 2010, 32, 1059–1062 CrossRef CAS PubMed.
- A. A. Homaei, R. Sariri, F. Vianello and R. Stevanato, Journal of Chemical Biology, 2013, 6, 185–205 CrossRef PubMed.
- S. Sulaiman, M. Mokhtar, M. Naim, A. Baharuddin and A. Sulaiman, Appl. Biochem. Biotechnol., 2015, 175, 1817–1842 CrossRef CAS PubMed.
- Z.-G. Wang, L.-S. Wan, Z.-M. Liu, X.-J. Huang and Z.-K. Xu, J. Mol. Catal. B: Enzym., 2009, 56, 189–195 CrossRef CAS.
- M. Misson, H. Zhang and B. Jin, J. R. Soc., Interface, 2015, 12, 20140891 CrossRef PubMed.
- N. R. Mohamad, N. H. C. Marzuki, N. A. Buang, F. Huyop and R. A. Wahab, Biotechnol. Biotechnol. Equip., 2015, 29, 205–220 CrossRef CAS PubMed.
- C. Tang, C. D. Saquing, S. W. Morton, B. N. Glatz, R. M. Kelly and S. A. Khan, ACS Appl. Mater. Interfaces, 2014, 6, 11899–11906 CAS.
- P. E. Erden and E. Kılıç, Talanta, 2013, 107, 312–323 CrossRef CAS PubMed.
- C. Chen, Q. Xie, D. Yang, H. Xiao, Y. Fu, Y. Tan and S. Yao, RSC Adv., 2013, 3, 4473–4491 RSC.
- C. R. Ispas, G. Crivat and S. Andreescu, Anal. Lett., 2012, 45, 168–186 CrossRef CAS.
- E. P. Cipolatti, M. J. A. Silva, M. Klein, V. Feddern, M. Manuel, C. Feltes, J. V. Oliveira, J. L. Ninow and D. d. Oliveira, J. Mol. Catal. B: Enzym., 2014, 99, 56–67 CrossRef CAS.
- S. K. Arya, M. Datta and B. D. Malhotra, Biosens. Bioelectron., 2008, 23, 1083–1100 CrossRef CAS PubMed.
- A. A. Ansari, M. Alhoshan, M. S. Alsalhi and A. S. Aldwayyan, Nanostructured Metal Oxides Based Enzymatic Electrochemical Biosensors, 2010 Search PubMed.
- X. Shi, W. Gu, B. Li, N. Chen, K. Zhao and Y. Xian, Microchim. Acta, 2014, 181, 1–22 CrossRef CAS.
- A. Senthamizhan, B. Balusamy and T. Uyar, Anal. Bioanal. Chem., 2015, 1–22 Search PubMed.
- G. Oskam, J. Sol-Gel Sci. Technol., 2006, 37, 161–164 CrossRef CAS.
- B. Ketterer, J. Arbiol and A. Fontcuberta i Morral, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 245327 CrossRef.
- P. Ohlckers and P. Pipinys, J. Appl. Phys., 2011, 109, 083713 CrossRef.
- A. G. Macedo, R. A. S. Ferreira, D. Ananias, M. S. Reis, V. S. Amaral, L. D. Carlos and J. Rocha, Adv. Funct. Mater., 2010, 20, 624–634 CrossRef CAS.
- Z. Zhong, M. C. Wingert, J. Strzalka, H.-H. Wang, T. Sun, J. Wang, R. Chen and Z. Jiang, Nanoscale, 2014, 6, 8283–8291 RSC.
- X. Chong, K.-J. Kim, E. Li, Y. Zhang, P. R. Ohodnicki, C.-H. Chang and A. X. Wang, PIE BiOS. International Society for Optics and Photonics, 2016, p. 970207 Search PubMed.
- A. E. Nel, L. Madler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS PubMed.
- B. Ding, M. Wang, X. Wang, J. Yu and G. Sun, Mater. Today, 2010, 13, 16–27 CrossRef CAS.
- S. Ramakrishna, K. Fujihara, W.-E. Teo, T. Yong, Z. Ma and R. Ramaseshan, Mater. Today, 2006, 9, 40–50 CrossRef CAS.
- A. Stafiniak, B. Boratyński, A. Baranowska-Korczyc, A. Szyszka, M. Ramiączek-Krasowska, J. Prażmowska, K. Fronc, D. Elbaum, R. Paszkiewicz and M. Tłaczała, Sens. Actuators, B, 2011, 160, 1413–1418 CrossRef CAS.
- Y. Zhang, Y. Wang, J. Jia and J. Wang, Sens. Actuators, B, 2012, 171–172, 580–587 CrossRef CAS.
- D. Liu, X. Zhang and T. You, ACS Appl. Mater. Interfaces, 2014, 6, 6275–6280 CAS.
- Y. Aykut, B. Pourdeyhimi and S. A. Khan, J. Phys. Chem. Solids, 2013, 74, 1538–1545 CrossRef CAS.
- A.-M. Azad, Mater. Sci. Eng., A, 2006, 435–436, 468–473 CrossRef.
- H. Xiang, Y. Long, X. Yu, X. Zhang, N. Zhao and J. Xu, CrystEngComm, 2011, 13, 4856–4860 RSC.
- M. Zhi, A. Manivannan, F. Meng and N. Wu, J. Power Sources, 2012, 208, 345–353 CrossRef CAS.
- C. Shao, H. Guan, Y. Liu, J. Gong, N. Yu and X. Yang, J. Cryst. Growth, 2004, 267, 380–384 CrossRef CAS.
- R. Ab Kadir, Z. Li, A. Z. Sadek, R. Abdul Rani, A. S. Zoolfakar, M. R. Field, J. Z. Ou, A. F. Chrimes and K. Kalantar-zadeh, J. Phys. Chem. C, 2014, 118, 3129–3139 CAS.
- Y. Liu, S. Sagi, R. Chandrasekar, L. Zhang, N. E. Hedin and H. Fong, J. Nanosci. Nanotechnol., 2008, 8, 1528–1536 CrossRef CAS PubMed.
- V. Aravindan, P. Suresh Kumar, J. Sundaramurthy, W. C. Ling, S. Ramakrishna and S. Madhavi, J. Power Sources, 2013, 227, 284–290 CrossRef CAS.
- H. Guan, C. Shao, S. Wen, B. Chen, J. Gong and X. Yang, Mater. Chem. Phys., 2003, 82, 1002–1006 CrossRef CAS.
- W. Zheng, Z. Li, H. Zhang, W. Wang, Y. Wang and C. Wang, Mater. Res. Bull., 2009, 44, 1432–1436 CrossRef CAS.
- S. H. Nam, H.-S. Shim, Y.-S. Kim, M. A. Dar, J. G. Kim and W. B. Kim, ACS Appl. Mater. Interfaces, 2010, 2, 2046–2052 CAS.
- Y. Wang, Y. Li, G. Sun, G. Zhang, H. Liu, J. Du, S. Yang, J. Bai and Q. Yang, J. Appl. Polym. Sci., 2007, 105, 3618–3622 CrossRef CAS.
- Y. Ding, Y. Liu, L. Zhang, Y. Wang, M. Bellagamba, J. Parisi, C. M. Li and Y. Lei, Electrochim. Acta, 2011, 58, 209–214 CrossRef CAS.
- Q. Guo, J. Huang, P. Chen, Y. Liu, H. Hou and T. You, Sens. Actuators, B, 2012, 163, 179–185 CrossRef CAS.
- N. Rodthongkum, N. Ruecha, R. Rangkupan, R. W. Vachet and O. Chailapakul, Anal. Chim. Acta, 2013, 804, 84–91 CrossRef CAS PubMed.
- Y. Ding, Y. Liu, J. Parisi, L. Zhang and Y. Lei, Biosens. Bioelectron., 2011, 28, 393–398 CrossRef CAS PubMed.
- A. Macagnano, E. Zampetti and E. Kny, Electrospinning for high performance sensors, 2015 Search PubMed.
- Z. Zhao, W. Lei, X. Zhang, B. Wang and H. Jiang, Sensors, 2009, 10, 1216–1231 CrossRef PubMed.
- M. Ahmad, C. Pan, Z. Luo and J. Zhu, J. Phys. Chem. C, 2010, 114, 9308–9313 CAS.
- J. Wu and F. Yin, Sens. Actuators, B, 2013, 185, 651–657 CrossRef CAS.
- M. Arvand, S. Tajyani and N. Ghodsi, Mater. Sci. Eng., C, 2015, 46, 325–332 CrossRef CAS PubMed.
- H. Tang, F. Yan, Q. Tai and H. L. W. Chan, Biosens. Bioelectron., 2010, 25, 1646–1651 CrossRef CAS PubMed.
- N. Zhang, Y. Deng, Q. Tai, B. Cheng, L. Zhao, Q. Shen, R. He, L. Hong, W. Liu, S. Guo, K. Liu, H.-R. Tseng, B. Xiong and X.-Z. Zhao, Adv. Mater., 2012, 24, 2756–2760 CrossRef CAS PubMed.
- R. Ramasamy, K. Ramachandran, G. G. Philip, R. Ramachandran, H. A. Therese and G. Gnana kumar, RSC Adv., 2015, 5, 76538–76547 RSC.
- H.-q. Liu, X.-l. Yu, B. Cai, S.-j. You, Z.-b. He, Q.-q. Huang, L. Rao, S.-s. Li, C. Liu, W.-w. Sun, W. Liu, S.-s. Guo and X.-z. Zhao, Appl. Phys. Lett., 2015, 106, 093703 CrossRef.
- X. Xiao, Y. Song, H. Liu, M. Xie, H. Hou, L. Wang and Z. Li, J. Mater. Sci., 2013, 48, 4843–4850 CrossRef CAS.
- T. H. Tran and V. T. Nguyen, Int. Scholarly Res. Not., 2014, 2014, 14 Search PubMed.
- W. Wang, L. Zhang, S. Tong, X. Li and W. Song, Biosens. Bioelectron., 2009, 25, 708–714 CrossRef CAS PubMed.
- G. Liu, B. Zheng, Y. Jiang, Y. Cai, J. Du, H. Yuan and D. Xiao, Talanta, 2012, 101, 24–31 CrossRef CAS PubMed.
- M. Gahlert, T. Gudehus, S. Eichhorn, E. Steinhauser, H. Kniha and W. Erhardt, Clin. Oral Implants Res., 2007, 18, 662–668 CrossRef CAS PubMed.
- M. Andreiotelli, H. J. Wenz and R.-J. Kohal, Clin. Oral Implants Res., 2009, 20, 32–47 CrossRef PubMed.
- H. Wang, Y. Duan and W. Zhong, ACS Appl. Mater. Interfaces, 2015, 7, 26414–26420 CAS.
- H. Wang, Y. She, C. Chu, H. Liu, S. Jiang, M. Sun and S. Jiang, J. Mater. Sci., 2015, 50, 5068–5078 CrossRef CAS.
- B. Yuan, C. Xu, D. Deng, Y. Xing, L. Liu, H. Pang and D. Zhang, Electrochim. Acta, 2013, 88, 708–712 CrossRef CAS.
- B. Wang, S. Gu, Y. Ding, Y. Chu, Z. Zhang, X. Ba, Q. Zhang and X. Li, Analyst, 2013, 138, 362–367 RSC.
- S. Marx, M. V. Jose, J. D. Andersen and A. J. Russell, Biosens. Bioelectron., 2011, 26, 2981–2986 CrossRef CAS PubMed.
- V. Vamvakaki, K. Tsagaraki and N. Chaniotakis, Anal. Chem., 2006, 78, 5538–5542 CrossRef CAS PubMed.
- J. Huang, Y. Liu and T. You, Anal. Methods, 2010, 2, 202–211 RSC.
- X. Tang, Y. Liu, H. Hou and T. You, Talanta, 2010, 80, 2182–2186 CrossRef CAS PubMed.
- D. Li, P. Lv, J. Zhu, Y. Lu, C. Chen, X. Zhang and Q. Wei, Sensors, 2015, 15, 29419 CrossRef CAS PubMed.
- X. Wang, C. Drew, S.-H. Lee, K. J. Senecal, J. Kumar and L. A. Samuelson, Nano Lett., 2002, 2, 1273–1275 CrossRef CAS.
- D. Kim and D. Kang, Sensors, 2008, 8, 6605 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1170 CrossRef CAS PubMed.
- J. A. Howarter and J. P. Youngblood, Langmuir, 2006, 22, 11142–11147 CrossRef CAS PubMed.
- P. Silberzan, L. Leger, D. Ausserre and J. J. Benattar, Langmuir, 1991, 7, 1647–1651 CrossRef CAS.
- J. Y. Huang, M. G. Zhao and Z. Z. Ye, Adv. Mater. Res., 2014, 950, 3–6 CrossRef.
- Y. Ding, Y. Wang, L. Zhang, H. Zhang, C. M. Li and Y. Lei, Nanoscale, 2011, 3, 1149–1157 RSC.
- M. Arvand, N. Ghodsi and M. Â. A. Zanjanchi, Biosens. Bioelectron., 2016, 77, 837–844 CrossRef CAS PubMed.
- M. Arvand and N. Ghodsi, Sens. Actuators, B, 2014, 204, 393–401 CrossRef CAS.
- W. Wang, Z. Li, W. Zheng, J. Yang, H. Zhang and C. Wang, Electrochem. Commun., 2009, 11, 1811–1814 CrossRef CAS.
- J. Shen, X. Yang, Y. Zhu, H. Kang, H. Cao and C. Li, Biosens. Bioelectron., 2012, 34, 132–136 CrossRef CAS PubMed.
- D. Xu, L. Luo, Y. Ding and P. Xu, Anal. Biochem., 2015, 489, 38–43 CrossRef CAS PubMed.
- L. Tang, Y. Tong, R. Zheng, W. Liu, Y. Gu, C. Li, R. Chen and Z. Zhang, Sens. Actuators, B, 2014, 203, 95–101 CrossRef CAS.
- G. Xu, S. Zhang, Q. Zhang, L. Gong, H. Dai and Y. Lin, Sens. Actuators, B, 2016, 222, 707–713 CrossRef CAS.
- J. Liu, J. Shen, M. Li and L.-P. Guo, Chin. Chem. Lett., 2015, 26, 1478–1484 CrossRef CAS.
- S.-j. Kim, Y. K. Cho, C. Lee, M. H. Kim and Y. Lee, Biosens. Bioelectron., 2016, 77, 1144–1152 CrossRef CAS PubMed.
- M. A. Ali, K. Mondal, Y. Jiao, S. Oren, Z. Xu, A. Sharma and L. Dong, ACS Appl. Mater. Interfaces, 2016, 8, 20570–20582 CAS.
- Y. Zhang, S. Liu, Y. Li, D. Deng, X. Si, Y. Ding, H. He, L. Luo and Z. Wang, Biosens. Bioelectron., 2015, 66, 308–315 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |








