Triphenylamine based lab-on-a-molecule for the highly selective and sensitive detection of Zn2+ and CN− in aqueous solution†
Shichao Sun‡
a,
Qinghai Shu‡*a,
Pengchao Lin‡a,
Yanyue Li‡a,
Shaohua Jin‡a,
Xin Chen‡*b and
Dequan Wang‡*c
aSchool of Materials Science & Engineering, Beijing Institute of Technology, Beijing, China. E-mail: qhshu121@bit.edu.cn
bState Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, China. E-mail: chenxin@sysucc.org.cn; Tel: +86-20-87343193
cInstitute of Theoretical Chemistry, Jilin University, Changchun, China. E-mail: dequan_wang@jlu.edu.cn
First published on 21st September 2016
Abstract
Simple dual sensing of Zn2+ and CN− was reported for the first time using the triphenylamine based lab-on-a-molecule TATP in both UV-Vis and fluorescence channels over other tested ions. Meanwhile, the sensing mechanism for both title ions was further studied using NMR titration and DFT calculations.
The selective and sensitive detection of ions has emerged as an area of great interest in biological, chemical and environmental processes. However, most of the reported ion sensors are effective only in the selective recognition of one particular analyte due to specific structural properties. Therefore, the development of a lab-on-a-molecule1–11 which has multiple recognition capability12–15 in a competitive fashion is still a challenging task.
Among metal ions, Zn2+ is the second most abundant essential trace element in the human body.16 It plays an important mediation role in lots of physiological processes, including the regulation of gene expression and apoptosis, and acts as a co-factor in metalloenzyme catalysis and neurotransmission.17
On the other hand, cyanide is one of the most concerning anions due to its high toxicity18–20 and reactivity. It is extensively produced in large quantities and applied in gold mining, plastic production and other industrial activities. Therefore, the detection of Zn2+ and CN− are of particular concern in many areas. Although some probes have been reported for the detection of either Zn2+ (ref. 21–31 and 49–51) or CN−,32–41,52,53 to the best of our knowledge, a simple sensor that can detect both Zn2+ and CN− competitively has rarely been reported.
As cyanide shows high activity for nucleophilic addition to double bonds, the easily constructed reaction site C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N was herein utilized for the recognition of cyanide. Further, the isomerization of C
N was herein utilized for the recognition of cyanide. Further, the isomerization of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N54 could be used to detect metal ions via the CHEF effect.42–44 Thus we designed a new probe TATP with well structured triphenylamine linked with C
N54 could be used to detect metal ions via the CHEF effect.42–44 Thus we designed a new probe TATP with well structured triphenylamine linked with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N as the recognition receptor, which cooperated with a phenolic hydroxyl group to form another recognition site for metal ions. As a result, TATP demonstrated excellent sensing behaviour towards Zn2+ and CN− among a series of cations and anions, compared with recently reported probes (Table 1).
N as the recognition receptor, which cooperated with a phenolic hydroxyl group to form another recognition site for metal ions. As a result, TATP demonstrated excellent sensing behaviour towards Zn2+ and CN− among a series of cations and anions, compared with recently reported probes (Table 1).
| Probes for zinc ions | Probes for cyanide | ||||
|---|---|---|---|---|---|
| Compound | LOD | Ref. | Compound | LOD | Ref. |
| TATP in our work | 14 nM | — | TATP in our work | 0.37 μM | — |
 |
6.3 × 10−11 M | 22 |  |
2.8 × 10−7 M | 33 |
 |
7.25 × 10−7 M | 24 |  |
1.5 μM | 34 |
 |
3.08 × 10−7 M | 24 |  |
0.478 μM | 35 |
 |
0.01 μM | 25 | 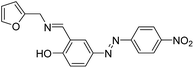 |
1.66 μM | 35 |
 |
3.2 μM | 49 |  |
3.2 × 10−7 M | 38 |
 |
4.6 μM | 50 |  |
1.6 μM | 52 |
 |
5.4 μM | 51 | 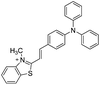 |
37 nM | 53 |
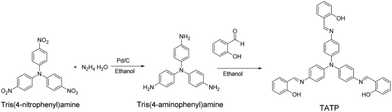
As shown in Fig. 1, TATP could be readily prepared through the reduction of tris(4-nitrophenyl)amine, followed by aldimine condensation with salicylide. The structure of TATP was confirmed using NMR, mass spectroscopy and elementary analysis. A crystal of TATP (Fig. 2), suitable for single-crystal X-ray diffraction, was obtained through slow evaporation from a mixture solution (ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane = 4
dichloromethane = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at room temperature, and displayed as a monoclinic crystal system with the space group P21/c.
1, v/v) at room temperature, and displayed as a monoclinic crystal system with the space group P21/c.
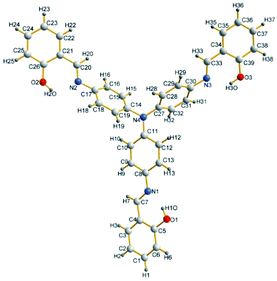 | ||
| Fig. 2 View of the crystal structure of TATP. Color code: C, gray; H, white; N, blue; O, red. The CCDC deposition number: 1476312. | ||
Results and discussion
Selective cation sensing of Zn2+ using TATP
The UV-Vis and fluorescence properties of TATP were investigated in 0.1 M Tris-ClO4 buffer solution (pH = 7.24, DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer = 1
buffer = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) by in the presence of the following various metal ions (as perchlorate salts): Ag+, Ba2+, Ca2+, Cd2+, Co2+, Cu2+, Hg2+, Ni2+, Pb2+ and Zn2+. Fig. S4a† shows that there was no significant variation in the absorption spectra of TATP for most of the added cations except for Zn2+ which induced a red shift from 412 nm to 425 nm.
2, v/v) by in the presence of the following various metal ions (as perchlorate salts): Ag+, Ba2+, Ca2+, Cd2+, Co2+, Cu2+, Hg2+, Ni2+, Pb2+ and Zn2+. Fig. S4a† shows that there was no significant variation in the absorption spectra of TATP for most of the added cations except for Zn2+ which induced a red shift from 412 nm to 425 nm.
From the emission properties of TATP (Fig. S4b†), it was shown to exhibit prominent fluorescence enhancement at 565 nm upon the addition of Zn2+, which could be attributed to the formation of a rigid system after binding with Zn2+, causing the chelation-enhanced fluorescence (CHEF) effect42–44 that inhibits C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation. This also can be verified through the small red shift of 13 nm in the UV-Vis spectra (Fig. S4a†) and the 1H NMR titration experiment (Fig. S12†), in which the sharp peak of –OH becomes gentle, suggesting that the intramolecular hydrogen bond turns to an intermolecular hydrogen bond due to metal complex formation.
N isomerisation. This also can be verified through the small red shift of 13 nm in the UV-Vis spectra (Fig. S4a†) and the 1H NMR titration experiment (Fig. S12†), in which the sharp peak of –OH becomes gentle, suggesting that the intramolecular hydrogen bond turns to an intermolecular hydrogen bond due to metal complex formation.
Consistent with the change in the fluorescence spectra, a solution of TATP underwent an immediate color change from colorless to yellow with Zn2+, indicating that TATP can serve as a ‘naked-eye’ Zn2+ indicator.
From the fluorescence titration profiles (Fig. 3a), the association constant for TATP–Zn2+ in 0.1 M Tris-ClO4 buffer solution (pH = 7.24, DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer = 1
buffer = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) was determined as 1.68 × 1019 M−2 using the Hill equation. A Job plot indicated a 1
2, v/v) was determined as 1.68 × 1019 M−2 using the Hill equation. A Job plot indicated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometric complexation of TATP with Zn2+ (Fig. S5†). The detection limit using TATP for the analysis of the Zn2+ ion was determined as 14 nM. Compared to well-known Zn2+ sensors, TATP shows a highly selective and sensitive response to Zn2+ over other various cations with a nanomolar detection limit.
3 stoichiometric complexation of TATP with Zn2+ (Fig. S5†). The detection limit using TATP for the analysis of the Zn2+ ion was determined as 14 nM. Compared to well-known Zn2+ sensors, TATP shows a highly selective and sensitive response to Zn2+ over other various cations with a nanomolar detection limit.
Further, the selectivity toward Zn2+ was ascertained through competition experiments. As shown in Fig. 3b, TATP was treated with 50 eq. of Zn2+ in the presence of other metal ions of the same concentration and no significant changes in the fluorescence intensities or emission wavelengths was found in the presence of other metal ions, indicating that the TATP–Zn2+ system was hardly affected by these coexistent metal ions. Thus, TATP can be used as a selective and sensitive fluorescence sensor for Zn2+ determination.
Selective anion sensing of CN− using TATP
The UV-Vis and fluorescence spectra (Fig. S6a†) of TATP were investigated in 0.1 M Tris-ClO4 buffer solution (pH = 7.24, DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer = 1
buffer = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) in the presence of the following various anions (as n-tetrabutylammonium salts): F−, AcO−, Cl−, Br−, I−, H2PO4−, PF6− and CN−. The selective response in the absorbance of TATP at 414 nm decreased and a new band at 512 nm emerged only in the presence of CN−, along with a solution color change from colorless to brown (Fig. S6b† inset), indicating an intramolecular charge transfer (ICT) mechanism45–47 from the clear isosbestic point at 461 nm between TATP and CN− upon the addition of excessive cyanide (Fig. S7†). Meanwhile, the strong emission quenching effect at 565 nm was only observed upon the addition of CN− (Fig. S6b†).
2, v/v) in the presence of the following various anions (as n-tetrabutylammonium salts): F−, AcO−, Cl−, Br−, I−, H2PO4−, PF6− and CN−. The selective response in the absorbance of TATP at 414 nm decreased and a new band at 512 nm emerged only in the presence of CN−, along with a solution color change from colorless to brown (Fig. S6b† inset), indicating an intramolecular charge transfer (ICT) mechanism45–47 from the clear isosbestic point at 461 nm between TATP and CN− upon the addition of excessive cyanide (Fig. S7†). Meanwhile, the strong emission quenching effect at 565 nm was only observed upon the addition of CN− (Fig. S6b†).
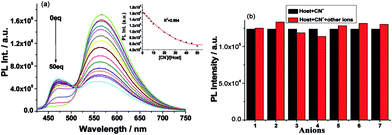
The recognition properties of TATP for CN− were further studied using absorption titration (Fig. S7†) and fluorescence titration (Fig. 4a) experiments. As shown in Fig. 4a, continuous emission quenching at 379 nm was observed after the addition of CN−. The Job plot for the binding between TATP and CN− exhibited 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometry (Fig. S9†), as depicted in Fig. 5. From the fluorescence titration data, the apparent association constant for TATP and CN− was determined as 1.7 × 103 using a nonlinear-fitting analysis of the change in the absorbance at 565 nm (R2 = 0.994),48 and the detection limit of TATP for the analysis of CN− was 5 μM based on the fluorescence titration experiments, and 0.37 μM based on UV-Vis absorption, which is lower than the WHO drinking water standard of 1.9 μM.34,38
3 stoichiometry (Fig. S9†), as depicted in Fig. 5. From the fluorescence titration data, the apparent association constant for TATP and CN− was determined as 1.7 × 103 using a nonlinear-fitting analysis of the change in the absorbance at 565 nm (R2 = 0.994),48 and the detection limit of TATP for the analysis of CN− was 5 μM based on the fluorescence titration experiments, and 0.37 μM based on UV-Vis absorption, which is lower than the WHO drinking water standard of 1.9 μM.34,38
The selectivity toward CN− was further ascertained using competition experiments. As shown in Fig. 4b, TATP was treated with 50 equivalents of CN− in the presence of other anions of the same concentration. Relatively low interference was observed in the detection of CN− in the presence of other anions. Thus TATP can be used as a selective fluorescent sensor toward CN− in the presence of most competing anions.
To make certain the sensing mechanism of TATP for CN−, 1H NMR titration experiments were performed as shown in Fig. S13.† The peak of –OH at 13.20 ppm disappeared rapidly after cyanide addition due to the deprotonation effect. Meanwhile, the peak of –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– at 9.00 ppm weakened and a new peak at around 10.20 ppm was raised which was considered to be –C–NH–, further proving the cyanide addition mechanism and thus demonstrating the destruction of the conjugation structure to cause the fluorescence quenching.
CH– at 9.00 ppm weakened and a new peak at around 10.20 ppm was raised which was considered to be –C–NH–, further proving the cyanide addition mechanism and thus demonstrating the destruction of the conjugation structure to cause the fluorescence quenching.
To get an insight into the electronic structures of TATP, TATP–CN− and TATP–Zn2+, DFT calculations were performed using the B3LYP/6-31G(d,p) basis set to understand the intermolecular interactions between the probe TATP and the ions (Fig. 6). The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) diagrams of TATP, TATP + CN− and TATP + Zn2+ indicate that internal charge transfer occurs during the anion recognition process.
In the case of TATP, the main molecular orbital (MO) contribution of the first lowest excited state was determined for the HOMO → LUMO transition, which was assigned to be an ICT band. In contrast, the cyanide addition and zinc binding in TATP caused a decrease in the HOMO-to-LUMO energy gap, which substantially resulted in a bathochromic shift, which supports the absorbance at longer wavelengths.
Finally, cellular imaging of TATP in the presence of the target ions was also carried out (Fig. 7). It could be found that TATP was aggregated in a quasi-water matrix instead of being taken into cells, which weakened its reaction with the ions. Nevertheless, a slight emission enhancement could be seen in the presence of zinc and cyanide ions. Compared with the results observed in solution, the ion recognition performance of the probe TATP was not so obvious because its water solubility is not good enough to perform biological experiments.
Experimental
Synthesis
Theoretical studies
The structural optimization and theoretical UV-Vis spectra of the probe TATP, TATP + Zn2+ and TATP + CN− were calculated using the computer program Gaussian 09W, by applying density functional theory (DFT) and time dependent DFT (TD-DFT) methods with a hybrid functional B3LYP using the basis sets 6-31G(d,p).Cellular imaging
The potential for cellular imaging with TATP was tested through the use of MCF-7 cancer cells. 2.0 mL of MCF-7 cells in DMEM medium at an initial density of 4 × 104 cell per mL were seeded in each dish and cultured at 37 °C for 24 h under a humidified atmosphere containing 5% CO2. A dispersion of TATP was prepared in DMEM medium (with 1% DMF) at a concentration of 10 μg mL−1 (first dissolved in DMF at 1 mg mL−1 and then diluted with DMEM medium to the target concentration). Cells were cultured with the dispersion of TATP for 6 h, then washed three times with PBS to remove the free materials, then cultured with a solution of Zn2+ (15 μg mL−1) or CN− (100 μg mL−1) for 30 min, and then washed three times with PBS to remove the free materials. Finally, the samples were observed using confocal laser fluorescence microscopy (TCS SP5II, Leica, Germany).Conclusions
In summary, we have successfully designed and synthesized a simple lab-on-a-molecule TATP probe, capable of sensing both cations and anions in aqueous solution. TATP exhibited excellent selectivity and sensitivity towards Zn2+ and CN− through inducing instantaneous solution color change, visible by the naked eye, and dramatic emissive responses, even in the presence of other ions. The binding model between the host and guests was demonstrated using Job's plot, NMR titration and DFT calculations, suggesting that the use of TATP to sense Zn2+ and CN− was achieved through metal–ligand coordination and nucleophilic attack mechanisms, respectively. The detection limits for Zn2+ and CN− using TATP were determined as 14 nm and 0.37 μM, respectively. On the basis of the results, we believe that the receptor TATP will offer important guidance in the development of a single receptor for recognizing both cations and anions in aqueous solution.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (21507005) and the Excellent Young Scholar Research Fund of Beijing Institute of Technology of China (3090012331542). We also acknowledge the assistance in the cell imaging tests from Ms Shan Sun of the Ningbo Institute of Industrial Technology, Chinese Academy of Sciences.Notes and references
- K. Chen, Q. Shu and M. Schmittel, Chem. Soc. Rev., 2015, 44, 136–160 RSC.
- M. Schmittel and Q. Shu, Chem. Commun., 2012, 48, 2707–2709 RSC.
- K. Chen and M. Schmittel, Analyst, 2013, 138, 6742–6745 RSC.
- K. Chen and M. Schmittel, Chem. Commun., 2014, 50, 5756–5759 RSC.
- D. C. Magri, G. J. Brown, G. D. Mcclean and A. Prasanna de Silva, J. Am. Chem. Soc., 2006, 128, 4950 CrossRef CAS PubMed.
- D. C. Magri, M. C. Fava and C. J. Mallia, Chem. Commun., 2014, 50, 1009–1011 RSC.
- K. Chen, J. W. Bats and M. Schmittel, Inorg. Chem., 2013, 52, 12863–12865 CrossRef CAS PubMed.
- Q. Shu, B. Lars and S. Michael, Aqueous Solution, Inorg. Chem., 2012, 51, 13123–13127 CrossRef CAS PubMed.
- B. Shan, Y. Liu, R. Shi, S. Jin, L. Li, S. Chen and Q. Shu, RSC Adv., 2015, 5, 96665–96669 RSC.
- L. Wan, Q. Shu, J. Zhu, S. Jin, N. Li, X. Chen and S. Chen, Talanta, 2016, 152, 39–44 CrossRef CAS PubMed.
- S. A. Lee, G. R. You, Y. W. Choi, H. Y. Jo, A. R. Kim, I. Noh, S. J. Kim, Y. Kim and C. Kim, Dalton Trans., 2014, 43, 6650–6659 RSC.
- M. Schmittel and H. W. Lin, Angew. Chem., Int. Ed., 2007, 46, 893–896 CrossRef CAS PubMed.
- L. Liu and H. Lin, Anal. Chem., 2014, 86, 8829–8834 CrossRef CAS PubMed.
- K. Jiang, S. Sun, L. Zhang, Y. Wang, C. Cai and H. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 23231–23238 CAS.
- Y. Leng, S. Qian, Y. Wang, C. Lu, X. Ji, Z. Lu and H. Lin, Sci. Rep., 2016, 6, 25354–25362 CrossRef CAS PubMed.
- Y. Huang, Q. Lin, J. Wu and N. Fu, Dyes Pigm., 2013, 99, 699–704 CrossRef CAS.
- H. Wang, C. L. Sun, Y. H. Yue, F. F. Yin, J. Q. Jiang, H. R. Wu and H.-L. Zhang, Analyst, 2013, 138, 5576–5579 RSC.
- B. Vennesland, E. E. Comm, C. J. Knownles, J. Westly and F. Wissing, Cyanide in Biology, Academic Press, London, 1981 Search PubMed.
- J. Y. Noh, I. H. Hwang, H. Kim, E. J. Song, K. B. Kim and C. Kim, Bull. Korean Chem. Soc., 2013, 34, 1985–1989 CrossRef CAS.
- G. J. Park, I. H. Hwang, E. J. Song, H. Kim and C. Kim, Tetrahedron, 2014, 70, 2822–2828 CrossRef CAS.
- C. Alberto Huerta-Aguilar, T. Pandiyan, P. Raj, N. Singh and R. Zanella, Sens. Actuators, B, 2016, 223, 59–67 CrossRef.
- A. A. A. Aziz, S. H. Seda and S. F. Mohammed, Sens. Actuators, B, 2016, 223, 566–575 CrossRef.
- R. Bhowmick, R. Alam, T. Mistri, K. K. Das, A. Katarkar, K. Chaudhuri and M. Ali, RSC Adv., 2016, 6, 11388–11399 RSC.
- A. Jimenez-Sanchez, B. Ortiz, V. Ortiz Navarrete, N. Farfan and R. Santillan, Analyst, 2015, 140, 6031–6039 RSC.
- G. J. Park, J. J. Lee, G. R. You, L. Nguyen, I. Noh and C. Kim, Sens. Actuators, B, 2016, 223, 509–519 CrossRef CAS.
- C. Patra, A. K. Bhanja, C. Sen, D. Ojha, D. Chattopadhyay, A. Mahapatra and C. Sinha, Sens. Actuators, B, 2016, 228, 287–294 CrossRef CAS.
- K. Ponnuvel, M. Kumar and V. Padmini, Sens. Actuators, B, 2016, 227, 242–247 CrossRef CAS.
- F. Qian, C. Zhang, Y. Zhang, W. He, X. Gao, P. Hu and Z. Guo, J. Am. Chem. Soc., 2009, 131, 1460–1468 CrossRef CAS PubMed.
- J. C. Qin, L. Fan and Z. Y. Yang, Sens. Actuators, B, 2016, 228, 156–161 CrossRef CAS.
- Y. Jin, S. Wang, Y. Zhang and B. Song, Sens. Actuators, B, 2015, 225, 167–173 CrossRef.
- N. Khairnar, K. Tayade, S. K. Sahoo, B. Bondhopahyay, A. Basu, J. Singh, N. Singh, V. Gite and A. Kuwar, Dalton Trans., 2015, 44, 2097–2102 RSC.
- E. G. Langeroudi, G. F. Petre, A. Azizi, E. Proulx and F. Larachi, J. Sep. Sci., 2011, 34, 1568–1573 CrossRef CAS PubMed.
- J. Li, W. Wei, X. Qi, G. Zuo, J. Fang and W. Dong, Sens. Actuators, B, 2016, 228, 330–334 CrossRef CAS.
- A. K. Mahapatra, K. Maiti, R. Maji, S. K. Manna, S. Mondal, S. S. Ali and S. Manna, RSC Adv., 2015, 5, 24274–24280 RSC.
- N. Maurya, S. Bhardwaj and A. K. Singh, Sens. Actuators, B, 2016, 229, 483–491 CrossRef CAS.
- T. Noipa, T. Tuntulani and W. Ngeontae, Talanta, 2013, 105, 320–326 CrossRef CAS PubMed.
- L. Shang, L. Zhang and S. Dong, Analyst, 2009, 134, 107–113 RSC.
- B. Shi, P. Zhang, T. Wei, H. Yao, Q. Lin and Y. Zhang, Chem. Commun., 2013, 49, 7812–7814 RSC.
- Y. Singh and T. Ghosh, Talanta, 2016, 148, 257–263 CrossRef CAS PubMed.
- L. Wang, G. Fang and D. Cao, Sens. Actuators, B, 2015, 221, 63–74 CrossRef CAS.
- G. R. You, G. J. Park, S. A. Lee, Y. W. Choi, Y. S. Kim, J. J. Lee and C. Kim, Sens. Actuators, B, 2014, 202, 645–655 CrossRef CAS.
- M. Shellaiah, Y. H. Wu and H. C. Lin, Analyst, 2013, 138, 2931–2942 RSC.
- J. C. Qin, T. R. Li, B. D. Wang, Z. Y. Yang and L. Fan, Spectrochim. Acta, Part A, 2014, 133, 38–43 CrossRef CAS PubMed.
- S. Kim, J. Y. Noh, K. Y. Kim, J. H. Kim, H. K. Kang, S. W. Nam, S. H. Kim, S. Park, C. Kim and J. Kim, Inorg. Chem., 2012, 51, 3597–3602 CrossRef CAS PubMed.
- N. Kumari, S. Jha and S. Bhattacharya, J. Org. Chem., 2011, 76, 8215–8222 CrossRef CAS PubMed.
- P. Xie, F. Guo, S. Yang, D. Yao, G. Yang and L. Xie, J. Fluoresc., 2013, 24, 473–480 CrossRef PubMed.
- L. Li, F. Liu and H. W. Li, Spectrochim. Acta, Part A, 2011, 79, 1688–1692 CrossRef CAS PubMed.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305–1323 RSC.
- S. Goswami, A. K. Das and K. Aich, Analyst, 2013, 138, 4593–4598 RSC.
- S. Goswami, A. K. Das and B. Pankhira, Dalton Trans., 2014, 43, 12689–12697 RSC.
- S. Goswami, S. Maity and A. C. Maity, Tetrahedron Lett., 2014, 55, 5993–5997 CrossRef CAS.
- S. Goswami, A. Manna and S. Paul, Chem. Commun., 2013, 49, 2912–2914 RSC.
- S. Goswami, A. Manna and S. Paul, Tetrahedron Lett., 2013, 54, 1785–1789 CrossRef CAS.
- J. Wu, W. Liu and J. Ge, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1476312. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra17354c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |