Installing tungsten Fischer carbene complexes into a calixarene framework†
M. D'Acunto*a,
S. Tommasonea,
C. Talottaa,
G. Brancatellib,
S. Geremiab,
E. Vallettac,
F. Marino Merlod,
B. Macchic,
C. Gaetaa,
P. Neri*a and
A. Spinella*a
aDipartimento di Chimica e Biologia “A. Zambelli”, Università di Salerno, Via Giovanni Paolo II 132, I-84084 Fisciano, Salerno, Italy. E-mail: mdacunto@unisa.it; neri@unisa.it; spinella@unisa.it
bCentro di Eccellenza in Biocristallografia, Dipartimento di Scienze Chimiche e Farmaceutiche, Università, di Trieste, via L. Giorgieri 1, I-34127 Trieste, Italy
cDipartimento di Medicina dei Sistemi, Università di Roma Tor Vergata, Via Montpellier 1, I-00133 Roma, Italy
dDipartimento di Scienze Chimiche, Biologiche, Farmaceutiche e Ambientali, Università di Messina, Viale F. Stagno d'Al-contres 31, I-98166 Messina, Italy
First published on 3rd August 2016
Abstract
The synthesis of the first examples of calix[4]arene-based Fischer carbene complexes is reported here. Fischer carbene complex 2 is the key intermediate to reach uracil-like derivatives 3 and 4. X-ray and NMR studies revealed that the uracyl-like synthon gives rise to a supramolecular dimer. The organometallic calixarene complexes showed a promising cytotoxicity towards human tumor cell lines.
Introduction
Among the large number of supramolecular uses of calixarene macrocycles,1 their biological applications have attracted a special interest.2 In particular, some calixarene derivatives have shown interesting biological activities as DNA intercalators.3 On the other hand, the use of transition metal complexes as DNA interacting agents (e.g.: cis-platin and related compounds) has been widely exploited during the last few years and these derivatives have shown cytotoxic effects toward tumor cells.4 A survey of the literature reveals several examples of calix[4]arenes conjugated to organometallic complexes of different nature,5 but, to the best of our knowledge, calix[4]arene conjugated to organometallic Fischer carbene complexes6 are still unknown. Such derivatives could be interesting powerful synthetic intermediates for the preparation of novel compounds able to interact with DNA or its related enzyme machinery.Fischer carbene complexes6 are powerful reagents in organic synthesis.7 The original and most general procedure employed in the preparation of Fischer metal carbenes follows a one pot scheme consisting in a sequential in situ preparation of organolithium reagents (such as alkynyllithium derivatives), their subsequent addition to hexacarbonyl chromium or tungsten complexes8 affording acylmetalates, and finally the O-alkylation by hard alkylating agents such as trialkyloxonium tetrafluoroborates.9
Such (alkynyl)–(alkoxy) Fischer carbene complexes are key intermediates in a number of reaction pathways.7,10 In particular, they have been widely employed in the preparation of uracil fragments by means of reaction with N-alkylureas.11
The mechanism suggested involves a Michael-type nucleophilic attack followed by cyclisation. Of course, the use of unsymmetrical urea leads to the formation of two isomeric compounds.11b The regioselectivity of this process is driven by the bulkiness of the N-monoalkylurea employed, the favored regioisomer being C (Scheme 1).12 Uracil-like B and C compounds can be further elaborated through oxidative13 and base-mediated demetalation.14 Prompted by these considerations we have designed the calixarene-Fischer carbene derivatives 3 and 4 (Scheme 2) and herein we describe their synthesis, X-ray studies, and cytotoxic effects toward tumor cells lines.
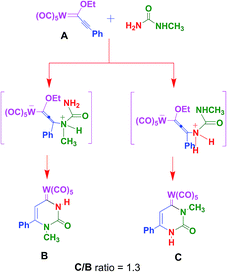 | ||
| Scheme 1 Mechanism and regiochemistry of the reaction of N-methylurea with (alkynyl)–(alkoxy)–carbene complex A. | ||
Results and discussion
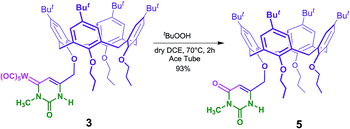
The synthesis of 3 and 4 is outlined in Scheme 2. In particular, the known alkynyl-calix[4]arene 1,15 was subjected to the Fischer one-pot scheme consisting of a sequential chemoselective lithiation at −78 °C by an in situ reaction with n-BuLi, trapping of the resulting 1-lithio-alkyne with tungsten hexacarbonyl affording the acylmetalate, and finally the O-alkylation by triethyloxonium tetrafluoroborate.8 In this way, the first calixarene derivative 2 containing an organometallic tungsten Fischer carbene complex was obtained in 20% yield, after column chromatography, with 50% recovery of the starting material. The above conversion (40%) can be considered a satisfactory result due to the complexity of the substrate and the potentially competitive formation of other lithiated products.16,17The incorporation of the carbene moiety in 2 was revealed by mass spectrometry and NMR spectroscopy. In particular, the carbene carbon, which is a strongly electrophilic center stabilized by π-donation from the oxygen, showed a diagnostic low-field 13C NMR signal at 285 ppm resembling those of carbenium ions.7 The presence of the axial CO ligand was evidenced by a signal at 206 ppm, while the other four equatorial CO resonated at 197 ppm. With the complex 2 in hands we decided to prepare the corresponding uracil derivatives.
Reaction with simple urea was unsuccessful, whereas the Michael-type conjugated addition followed by cyclisation of N-methylurea to the (alkynyl)–(alkoxy)–carbene 2 gave the two uracil-like regioisomers 3 and 4 (Scheme 2). As previously described, in this reaction the regioselectivity depends on the nucleophilicity and bulkiness of the urea substituent.12 In this case the main regioisomer 3, formed by the first nucleophilic attack of the unsubstituted nitrogen, was surprisingly, much more abundant than its isomer 4 with a ratio of 10/1. The explanation of this high regioselectivity can be found in the steric hindrance brought by the very bulky calixarene framework.
The correct regiochemistry for each isomer 3 and 4 was established by HMBC experiments. In fact, two correlations were seen in the spectrum of 3 at 4.22/240 and 4.22/148 ppm (Fig. 1) between the N-methyl protons and the carbene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) W and the amide C
W and the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbons, respectively, which are only compatible with two 3J long-range couplings due to its midway position. The other possible regioisomer 4 showed only one 3J long-range correlation between the N-methyl group and the amide C
O carbons, respectively, which are only compatible with two 3J long-range couplings due to its midway position. The other possible regioisomer 4 showed only one 3J long-range correlation between the N-methyl group and the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 3.88/148 ppm (Fig. S16†).
O at 3.88/148 ppm (Fig. S16†).
Single crystal X-ray analyses provided a further independent proof of the structure of 3 and 4. In the solid-state structure of the major isomer 3, the N-methyl group was located between the carbene and the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, and was pointing toward the exterior of the molecule, whereas the free amide NH was pointing toward the interior of the adjacent propylated oxygens giving an intramolecular H-bond (N⋯O 2.839(8) Å) with one of them (Fig. 2). The calix skeleton of 3 was found in a pinched cone conformation with the distal Ar-OPr rings (A and C, Fig. 2) almost parallel to each other, with a roughly orthogonal orientation with respect to the mean plane defined by the four bridging methylene carbon atoms (canting angles for A and C are 98.2(1) and 82.8(2)°), while the other ones B and D were more opened with canting angle values of 47(2) and 49.5(2)°.
O group, and was pointing toward the exterior of the molecule, whereas the free amide NH was pointing toward the interior of the adjacent propylated oxygens giving an intramolecular H-bond (N⋯O 2.839(8) Å) with one of them (Fig. 2). The calix skeleton of 3 was found in a pinched cone conformation with the distal Ar-OPr rings (A and C, Fig. 2) almost parallel to each other, with a roughly orthogonal orientation with respect to the mean plane defined by the four bridging methylene carbon atoms (canting angles for A and C are 98.2(1) and 82.8(2)°), while the other ones B and D were more opened with canting angle values of 47(2) and 49.5(2)°.
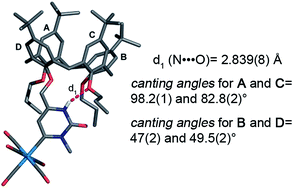 | ||
| Fig. 2 Solid state structure of calix[4]arene Fischer carbene complex 3. Co-crystallized solvent molecules and all hydrogen atoms, except for the NH involved in the H-bond, were omitted for clarity. | ||
As expected, the X-ray analysis performed on the minor regioisomer 4 confirmed the position of the N-methyl group in the uracil ring, and evidenced that it is now pointing toward the interior of the molecule. As observed in 3, also the cavity of 4 was found distorted with the distal Ar-OPr rings, A and C, almost orthogonal to the mean calixarene plane (dihedral angles 90.27(6) and 81.44(6)°, respectively, Fig. 3), and the B and D rings more tilted outwards the cavity (dihedral angles of 52.21(6) and 46.95(5)°, respectively, Fig. 3). On the other hand, the free amide NH is now directed toward the exterior of the molecule, and is involved in a symmetrical H-bond (N⋯O 2.833(2) Å, Fig. 3) with the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of another equivalent molecule. This synthon, typical of uracyl-like rings and described by the graph set R22(8), according to the Etter's rule,18 gives rise to a supramolecular calixarene dimer.
O group of another equivalent molecule. This synthon, typical of uracyl-like rings and described by the graph set R22(8), according to the Etter's rule,18 gives rise to a supramolecular calixarene dimer.
 | ||
| Fig. 3 Solid state structure of derivative 4 (left) and its solid-state self-assembled dimer (right). Hydrogen atoms, t-butyl and propyl groups were omitted for clarity. | ||
The presence in the solid state of a self-assembled dimer of 4, prompted us to investigate its association in solution by 1H NMR studies. Thus, it was found that the chemical shift of the amide NH at 10.22 ppm shifted upon dilution in the 0.87–0.46 mM range, consistently with the dimer formation (Fig. S21†). From these dilution experiments, a dimerization constant of 46 ± 5 M−1,19a was determined, which is in line with comparable values found for similar systems.19b The extension of these studies to regioisomer 4 indicated the absence of a measurable association as expected by the unfavorable relative geometry of the CO–NH group.
As a preliminary exploration of the synthetic potentialities of the calixarene Fischer carbene complexes, we decided to study the oxidation of the metal center. It is well known that several reagents (e.g. DMSO) used to oxidize Fischer carbene complexes, are often unsuccessful when applied on nitrogen substituted carbene complexes.20a,b In fact, oxidations of Fischer tungsten–carbene uracil complexes are easily performed using the Barluenga's protocol based on the use of fluoride ion20c or using t-butyl hydroperoxide (t-BuOOH). DMSO give no reaction at all at room temperature and low yields (with formation of byproducts due to degradation of the substrate) at higher temperature.13 In particular, the treatment of the major regioisomer 3 with t-BuOOH (Scheme 3) gives rise to its uracil analogue 5 in very high yield and with very high chemoselectivity.
In order to verify possible biological activities of the obtained calixarene Fischer carbene complexes, the in vitro effect on the metabolic activity of cancer cells was evaluated. Two different cell lines were used: CAL-27 (oral adenosquamous carcinoma cell line) and PC3 (human prostate cancer cell line). A clear stock solutions of complexes 2–4, and the demetalated derivative 5, in dimethyl sulfoxide, were prepared and used for their addition to the cell culture medium. After incubation for 24 h, the metabolic activity of the cells was evaluated through a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay, which measures the ability of a mitochondrial dehydrogenase enzyme from viable cells to cleave the tetrazolium rings. As a control, tumor cells were treated with a known chemotherapeutic drug, 7-ethyl-10-hydroxy-camptothecin (SN38), an active metabolite of irinotecan, topoisomerase I inhibitor. The results are expressed in Table 1 as IC50 values for the viability assays. Interestingly, tungsten carbene complex 2 was cytotoxic toward CAL 27 (24 ± 4 μM) and PC-3 (22 ± 3 μM) cell lines and its activities were comparable to those of a new cytotoxic calixarene derivative recently reported by our group.21 Calix[4]arene Fischer carbene complex 3 showed a slightly lower activity toward CAL-27 (55 ± 4 μM) and PC-3 (95 ± 14 μM) cell lines. Similar interesting results were obtained for derivative 4 with IC50 values of 41 ± 7 μM (CAL-27) and 42 ± 3 μM (PC-3). However, the corresponding derivative 5 showed no cytotoxic activities toward both of the assayed cell lines, suggesting a probable role of the organometallic moiety still present in derivatives 2–4.
Conclusions
In conclusion, we reported the first examples of calix[4]arenes conjugated to organometallic tungsten Fischer carbene complexes. Uracil-like complexes were obtained by Michael-type conjugated addition followed by cyclisation with N-methylurea with an unprecedented regioselectivity, likely driven by the bulkiness of the calixarene framework. An interesting H-bonded dimerization was evidenced both in the solid-state and in solution. Finally, interesting in vitro activities to inhibit the viability of two cancer cells line (CAL-27 and PC-3) were found, which augur well for further investigations on calixarene-based Fischer carbene complexes and on their biological activities.Acknowledgements
We thank Mr Stefano Caputo for the help given in the experimental work. The Italian MIUR (PRIN 20109Z2XRJ_006) for financial support and the Centro di Tecnologie Integrate per la Salute (Project PONa3_00138), Università di Salerno, for the 600 MHz NMR instrumental time are also gratefully acknowledged.Notes and references
- (a) C. D. Gutsche, Calixarenes, An Introduction, Royal Society of Chemistry, Cambridge, UK, 2008 Search PubMed; (b) Calixarenes and Beyond, ed. P. Neri, J. L. Sessler and M.-X., Wang, Springer, Switzerland, 2016, DOI:10.1007/978-3-319-31867-7; (c) F. Troisi, A. Russo, C. Gaeta, G. Bifulco and P. Neri, Tetrahedron Lett., 2007, 48, 7986 CrossRef CAS; (d) C. Talotta, C. Gaeta and P. Neri, Org. Lett., 2012, 14, 3104 CrossRef CAS PubMed; (e) C. Gaeta, T. Caruso, M. Mincolelli, F. Troisi, E. Vasca and P. Neri, Tetrahedron, 2008, 64, 5370 CrossRef CAS.
- (a) S. Fletcher and A. D. Hamilton, Curr. Opin. Chem. Biol., 2005, 9, 632 CrossRef CAS PubMed; (b) M. W. Peczuh and A. D. Hamilton, Chem. Rev., 2000, 100, 2479 CrossRef CAS PubMed; (c) See, ch. 22–24 in ref. 1b; (d) G. M. L. Consoli, G. Granata, E. Galante, F. Cunsolo and C. Geraci, Tetrahedron Lett., 2006, 47, 3245 CrossRef CAS; (e) G. M. L. Consoli, G. Granata, D. Garozzo, T. Mecca and C. Geraci, Tetrahedron Lett., 2007, 48, 7974 CrossRef CAS.
- (a) A. Rescifina, C. Zagni, P. G. Mineo, S. V. Giofrè, U. Chiacchio, S. Tommasone, C. Talotta, C. Gaeta and P. Neri, Eur. J. Org. Chem., 2014, 7605 CrossRef CAS; (b) G. Cafeo, G. Carbotti, A. Cuzzola, M. Fabbi, S. Ferrini, F. H. Kohnke, G. Papanikolaou, M. R. Plutino, C. Rosano and A. J. P. White, J. Am. Chem. Soc., 2013, 135, 2544 CrossRef CAS PubMed.
- (a) J. K. Barton, Comments Inorg. Chem., 1985, 3, 321 CrossRef CAS; (b) C. Allardyce, A. Dorcier, C. Scolaro and P. Dyson, Appl. Organomet. Chem., 2005, 19, 1–10 CrossRef CAS; (c) N. Farrell, Metal complexes as drugs and chemotherapeutic agents, in Comprehensive Coordination Chemistry II, vol. 9, pp. 809–840, ISBN 0-08-0443311 Search PubMed; (d) G. Palma, M. D'Aiuto, D. Rea, S. Bimonte, R. Lappano, M. R. Sinicropi, M. Maggiolini, P. Longo, C. Arra and C. Saturnino, Biochem. Pharmacol., 2014, 3, 2 Search PubMed.
- (a) J. W. Kueck, M. R. Anneser, B. Hofmann, A. Poethig, M. Cokoja and F. E. Kuehn, ChemSusChem, 2015, 8, 4056 CrossRef CAS PubMed; (b) Y. Visitaev, I. Goldberg and A. Vigalok, Inorg. Chem., 2013, 52, 6779 CrossRef CAS PubMed; (c) M. Sathiyendiran, C. C. Tsai, P. Thanasekaran, T.-T. Luo, C. I. Yang, G. H. Lee, S. M. Peng and K. L. Lu, Chem.–Eur. J., 2011, 17, 3343 CrossRef CAS PubMed; (d) A. Soriente, M. De Rosa, M. Fruilo, L. Lepore, C. Gaeta and P. Neri, Adv. Synth. Catal., 2005, 347, 816 CrossRef CAS.
- (a) E. O. Fischer and A. Maasböl, Angew. Chem., Int. Ed. Engl., 1964, 3, 580 CrossRef; (b) O. S. Mills and A. D. Redhouse, Angew. Chem., Int. Ed. Engl., 1965, 4, 1082 CrossRef CAS.
- K. H. Dötz and J. Stendel, Chem. Rev., 2009, 109, 3227 CrossRef PubMed.
- E. O. Fischer and A. Maasböl, Chem. Ber., 1967, 100, 2445 CrossRef.
- (a) E. O. Fischer and R. Aumann, Angew. Chem., Int. Ed. Engl., 1967, 6, 879 CrossRef; (b) E. O. Fischer and R. Aumann, Chem. Ber., 1968, 101, 954 CrossRef.
- (a) M. A. Sierra, Chem. Rev., 2000, 100, 3591 CrossRef CAS PubMed; (b) A. Meijere, H. Schirmer and M. Duetsch, Angew. Chem., Int. Ed., 2000, 39, 3964 CrossRef; (c) K. L. Faraon and W. D. Wulff, J. Am. Chem. Soc., 1988, 110, 8727 CrossRef; (d) K. S. Chan and W. D. Wulff, J. Am. Chem. Soc., 1986, 108, 5229 CrossRef CAS; (e) J. Barluenga, J. Santamaria and M. Tomàs, Chem. Rev., 2004, 104, 2259 CrossRef CAS PubMed.
- (a) R. Polo, J. M. Moretò, U. Schick and S. Ricart, Organometallics, 1998, 17, 2135 CrossRef CAS; (b) A. Spinella, T. Caruso, U. Pastore and S. Ricart, J. Organomet. Chem., 2003, 684, 266 CrossRef CAS.
- A. Artillo, G. Della Sala, M. De Santis, A. Llordes, S. Ricart and A. Spinella, J. Organomet. Chem., 2007, 692, 1277 CrossRef CAS.
- G. Della Sala, A. Artillo, S. Ricart and A. Spinella, J. Organomet. Chem., 2007, 692, 1623 CrossRef CAS.
- C. Bocchino, A. Carabellese, T. Caruso, G. Della Sala, S. Ricart and A. Spinella, J. Organomet. Chem., 2014, 749, 47 CrossRef CAS.
- S. Cecioni, R. Lalor, B. Blanchard, J. P. Praly, A. Imberty, S. E. Matthews and S. Vidal, Chem.–Eur. J., 2009, 15, 13232 CrossRef CAS PubMed.
- P. A. Scully, T. H. Hamilton and J. L. Bennett, Org. Lett., 2001, 3, 2741 CrossRef CAS PubMed.
- C. Fischer, W. Seichter and E. Weber, Beilstein J. Org. Chem., 2011, 7, 1602 CrossRef CAS PubMed.
- M. C. Etter, J. C. Mac Donald and J. Bernstein, Acta Crystallogr., Sect. B: Struct. Sci., 1990, 46, 256 CrossRef.
- (a) A. Zafar, S. J. Geib, Y. Hamuro, A. Carr and A. J. Hamilton, Tetrahedron, 2000, 56, 8419 CrossRef CAS; (b) S. C. Zimmerman and B. F. Duerr, J. Org. Chem., 1992, 57, 2215 CrossRef CAS.
- (a) D. Perdicchia, E. Licandro, S. Maiorana, B. Vandoni and C. Bandoli, Org. Lett., 2002, 4, 827 CrossRef CAS PubMed; (b) A. Lluch, L. Jordi, F. Sancez-Baeza, S. Ricart, F. Camps, A. Messeguer and J. M. Monetò, Tetrahedron Lett., 1992, 33, 3021 CrossRef CAS; (c) J. Barluenga, F. Andina, M. A. Fernandez-Rodriguez, P. Garcia-Garcia, I. Merino and E. Aguilar, J. Org. Chem., 2004, 69, 7352 CrossRef CAS PubMed.
- S. Tommasone, C. Talotta, C. Gaeta, L. Margarucci, M. C. Monti, A. Casapullo, B. Macchi, S. P. Prete, A. Ladeira De Araujo and P. Neri, Angew. Chem., Int. Ed., 2015, 54, 15405 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1D and 2D NMR spectra, details of the biological assays and dimerization studies and tables of crystal data. CCDC 1438070 and 1437666. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra17326h |
| This journal is © The Royal Society of Chemistry 2016 |