Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly stable and blue-emitting copper nanocluster dispersion prepared by magnetron sputtering over liquid polymer matrix†
Matteo
Porta
,
Mai Thanh
Nguyen
,
Yohei
Ishida
and
Tetsu
Yonezawa
 *
*
Division of Materials Science and Engineering, Faculty of Engineering, Hokkaido University, Kita 13 Nishi 8, Kita-ku, Sapporo, Hokkaido 060-8628, Japan. E-mail: testu@eng.hokudai.ac.jp
First published on 24th October 2016
Abstract
For the first time, magnetron sputtering of copper onto liquid was investigated for the preparation of stable blue-fluorescent copper nanoclusters. The fluorescent intensity increased linearly with an increase of mercaptan. Our finding casts light on the formation mechanism of metal photoluminescent nanoclusters via sputtering onto liquid.
Intensive emission and good stability are crucial in many applications such as bioimaging.1–9 Organic dyes are widely used in applications for single molecular imaging. However, they suffer rapid photofading,4,5 thus limiting longtime imaging. Semiconductor quantum dots have been developed with higher photo-stability and larger molar extinction coefficients compared to organic fluorophores. The semiconductor quantum dots often have a bigger size (several to some tens of nm), a higher tendency to aggregated, and even toxicity issues.4,5 Recently, metallic nanoclusters show interesting photoemission properties.1–10 They have smaller size (from few to hundreds of atoms), typically less than 2 nm, compared with semiconductor quantum dots.
Copper and copper composite nanomaterials are gaining increasing attention from fundamental and application basis. Copper is much cheaper than noble metals but provide high photoluminescence (PL) and its great ability to mix with other elements opens up an almost infinite range of uses for this metal. However, compared with gold or silver, making and stabilization of copper nanoclusters are more challenging tasks, due to the ease of oxidation. Therefore, more efforts are required from scientists in order to make the usage of copper nanoclusters a reality for applications. So far, many strategies have been developed to synthesize copper nanoclusters. Some of the most common methods include chemical reduction in solution11–14 and ultrasonic radiation15 to reduce copper ions and using organic capping molecules, biomolecules16 (e.g. DNA) to control the growth of the nanoclusters. These methods have some drawbacks such as using toxic reductants and influence of impurity on the nanocluster properties.
On the other hand, physical processes17–27 (cluster beam deposition,17 sputtering18–27) that do not need reducing agents, have been used to produce metal nanoclusters from the bulk metal source. The great advantage of these techniques over chemical reduction methods is the high purity of the nanoclusters that can be achieved, as the synthesis is done under vacuum conditions using a controlled atmosphere. However, in these techniques, solid substrates were often needed to support the bare metal nanoclusters.28,29 This limits the use of the metal nanoclusters for various in vivo bio-applications where well dispersed nanoclusters in a suitable solvent with surface functionalization are in demand. Recent progress in sputtering has shown that introducing a liquid substrate to replace the solid ones allowed for direct preparation of liquid dispersion of nanoclusters.20–26 The liquid substrates are based on molten salts and low vapour pressure liquids such as liquid polymer,22–26 vegetable oil,30 and ionic liquids.20,21,27 Moreover, when stabilizing molecules are added into the liquid substrate or sputtering atmosphere, it is possible to control particle size and properties (oxidation, surface functionalization, plasmonic/fluorescent properties). Previous works reported successful preparation of gold,20,22–24,27 silver,26 and plasmonic copper25 nanoparticles with controllable sizes. In particular, our previous report on matrix sputtering of copper shows that metallic copper nanoparticles with plasmonic properties could be prepared. The average sizes were controlled from 2–3 nm to 5.5–8 nm by varying the sputtering current. However, we found that the use of pentaerythritol ethoxylate (PEEL) as a liquid matrix with weak coordinating to copper could not fully protect copper nanoparticles in terms of particle size growth during sputtering into liquid and aggregation over time. Moreover, fluorescence copper nanoclusters could not be obtained.
In this paper, by using a stronger capping ligand, we report for the first time a synthesis of stable blue-emitting copper nanoclusters by sputtering copper onto a solution of poly(ethylene glycol) (PEG, MW = 600) and 11-mercaptoundecanoic acid (MUA). PEG and MUA were chosen, respectively, for a low vapour pressure and good capping ability with Cu to form active fluorescent copper nanoclusters. PL properties and possible formation pathways of copper nanoclusters with respect to the concentration of MUA will be discussed.
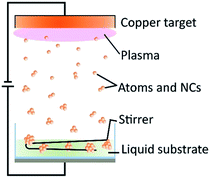
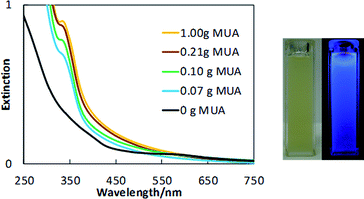
Copper nanoclusters were prepared via sputtering (Fig. 1) using various MUA amount (0–1.00 g, 0–0.514 mM) in PEG (7.00 g) in Ar at 40 °C. A metallic Cu target was placed 6 cm above the surface of the liquid. The sputtering of Cu onto the liquid substrate was performed for 60 min at sputtering current of 20 mA. Copper nanoclusters dispersed in MUA and PEG gave yellow dispersions (Fig. 2 and S1†). UV-vis extinction spectra of the resulting dispersions show no clear surface plasmonic resonance from metallic copper nanoparticles (Fig. 2). In the absence of MUA, higher absorption was observed in the UV-vis spectrum of the copper nanoparticles dispersed in PEG (both PEG and mixture of MUA and PEG do not absorb light in the visible region, Fig. S2†). This sample, however, did not show PL emission. When we observed the particle size (shown later in Fig. 4f) we found that copper particles have an average particle sizes of 2.6 ± 0.6 nm and showing a broader particle size distribution when compared with copper particles in samples sputtered using MUA and PEG. It is common for metal nanoparticles with a size larger than 2 nm not to have PL.31 Using MUA (0.07–1.00 g), a clear peak at 345 nm in UV-vis spectra (Fig. 2) was observed for all samples, indicating the formation of copper nanoclusters. These samples emitted blue light under UV irradiation (Fig. 2), which is consistent with the UV-vis results. We further confirmed that these samples composed of metallic copper capped with MUA on their surfaces using XPS measurements. Detailed XPS spectra, analysis and assignment were given in ESI, Fig. S3 and Table S1.†
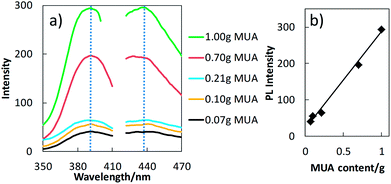
The PL excitation and emission spectra (Fig. 3a) of the copper nanocluster dispersions in MUA/PEG exhibit an emission peak maximum at 437 nm and an excitation peak maximum at 393 nm. These spectra belong to the copper nanoclusters since PEG and MUA themselves do not exhibit PL in this wavelength region (Fig. S4†). The peak maxima and the shape of the PL spectra of the nanocluster dispersions are identical among these samples, suggesting an identical nature (i.e. their sizes, composition) of the PL centres in these samples. However, the intensity of the peaks increased with an increase in the MUA amount used in the liquid substrate (Fig. 3b). This result indicates that the number of PL centres increases according to the amount of the stabilizing agent, MUA. Because all samples underwent the same sputtering parameters (i.e. time, current, substrate temperature and surface area, and target-liquid distance), except for the liquid matrix composition, the total volume of sputtered copper (number of copper atoms) in the liquid matrixes can be considered the same for all cases. Therefore, an increase in the number of PL centres indicates that the aggregation state (size and size distribution as well as the intra-clusters structure) of copper nanoclusters in the liquids is affected by the MUA concentration. Besides, considering the Jellium model for copper (eqn (SE1)†),10 based on the emission spectra of the dispersion, the size of the active PL centres was estimated and they would be composed by ∼15 atoms. With this number of copper atoms, the copper core size of the PL centres is less than 1 nm.31
The size and size distributions of MUA-stabilized copper nanoclusters were verified using TEM. The results (Fig. 4 and 5) show that samples containing 0.10 g of MUA or more, have particle sizes in the range of 1.6 ± 0.3 nm, the sample containing 0.07 g of MUA has particle size of 2.5 ± 0.3 nm, and sample without MUA has particle size of 2.6 ± 0.6 nm. The samples prepared in PEG matrix containing 0.07 g of MUA and PEG matrix without MUA show similar average size, but the former emitted fluorescence while the latter did not. In addition, the nanoclusters with size less than 1 nm were not found in the TEM images. However, all samples containing MUA emitted blue fluorescence, of which the active fluorescent centres of less than 1 nm. This suggested that the primary sputtered copper atoms and nanoclusters (as the active PL centres or to form the active PL centres) could experience further aggregation to certain sizes in the liquid substrate to form the nanoclusters as observed in Fig. 4. Moreover, MUA helped stabilize the active PL centres on their own and in the second aggregation state (nanoclusters containing these centres). The PL properties of these nanoclusters arise from the active PL centres stabilized with MUA, therefore all samples using MUA and PEG showed PL emission and excitation spectra in similar fashion. The higher amount of MUA, the larger number of the small active PL centres was stabilized and higher PL intensities were obtained. Further, we found that the fraction of nanoclusters with size less than 1.6 nm increased as a function of MUA amount (Fig. 5b), suggesting that a larger fraction of smaller nanoclusters was formed at higher MUA amount. The linear relation was observed for samples prepared using 0.10 g MUA or more and a sharp step between samples prepared using 0.07 g and that using 0.10 g MUA (both showed PL). Similar results were obtained when plotting the fraction of nanoclusters with size of 2.0 nm or less as a function of MUA amount (Fig. S5†). This indicated that nanoclusters may contain more than a single PL centre. In addition, with less MUA, the active PL centres can merge to form bigger nanoclusters, which are inactive PL. Similar phenomenon was reported in case of sputtered gold and copper nanoclusters in thiolate containing liquid matrixes.22–24,27,32
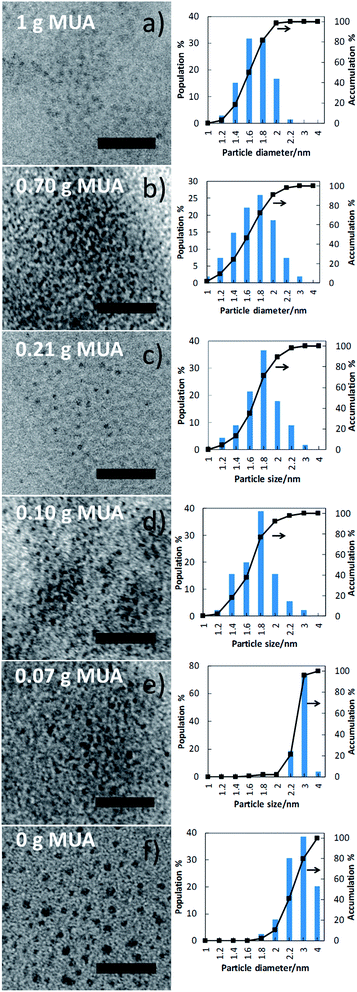 | ||
| Fig. 4 TEM images (left) and size distributions (right) of the sputtered samples with various amounts of MUA (0–1.00 g). All scale bars are 20 nm. | ||
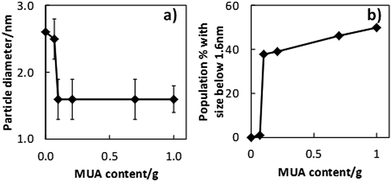 | ||
| Fig. 5 Nanoclusters average diameter and fraction of nanoclusters with size of 1.6 nm or less obtained using different amount of MUA. | ||
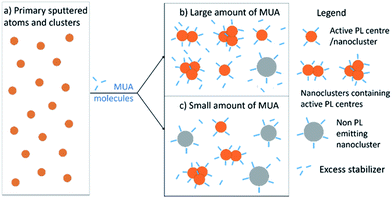
Fig. 6 illustrates the above mentioned formation mechanism of fluorescent copper nanoclusters. The primary sputtered copper atoms and clusters (Fig. 6a) are similar in all cases as soon as they were deposited onto MUA–PEG. These copper atoms and clusters were then dispersed in it and stabilized by MUA. Depending on the amount of MUA in the solution, the primary sputtered metal atoms and clusters aggregate to form nanoclusters with different size, size distribution, aggregation state (Fig. 6b and c). The active PL centres (small nanocluster, orange filled circle capped with MUA) are considered as the sub-unit of the fluorescent nanoclusters. The sizes of the photoluminescent nanoclusters depend on the number of the active PL centres in the nanoclusters (Fig. 6b). Despite the size difference among these resulting photoluminescent nanoclusters, they show similar PL properties. At low MUA amount, primary sputtered atoms and clusters merged to form large sized inactive nanoclusters (grey particles, Fig. 6c). Therefore, with the same number of copper atoms in the primary sputtered atoms and clusters, the number of the PL centres obtained using high amount of MUA are larger than that using lower amount of MUA (Fig. 6b and c). More MUA molecules contribute to the formation of more PL centres and photoluminescent nanoclusters as well as larger number of smaller sized nanoclusters. This proposed formation mechanism can explain the obtained identical PL excitation/emission maxima (one type of active PL centres), and the increase in PL intensity of the resulting dispersions for higher amount of MUA used.
Furthermore, we found that the blue emission PL of copper nanoclusters dispersed in MUA and PEG are highly stable with negligible photofading over time (Fig. S6†). This makes the method become promising for preparation of long lifetime fluorophores for various applications.
Conclusions
In conclusion, we demonstrated that our modified green and facile sputtering technique allowed for achieving highly stable blue-emitting copper nanoclusters dispersed in liquid. The PL intensity varied proportionally to the amount of MUA in the liquid substrate. The changes in MUA concentrations did not change the position of the PL peak maxima and particle size for MUA content above 0.10 g. The finding in this study indicates the formation of PL centres and their secondary aggregation to form photoluminescent nanoclusters.Acknowledgements
This work is partially supported by Hokkaido University. TY thanks a partial support from Murata Foundation and Canon Foundation.References
- B. Corain, G. Schmid and N. Toshima, Metal Nanoclusters in Catalysis and Materials Science: the Issue of Size Control, Elsevier B. V., 2008 Search PubMed.
- O. S. Wolfbeis, Chem. Soc. Rev., 2015, 44, 4743 RSC.
- C. Wang, L. Ling, Y. Yao and Q. Song, Nano Res., 2015, 8, 1975 CrossRef CAS.
- Z. Li, Q. Sun, Y. Zhu, B. Tan, Z. P. Xu and S. X. Dou, J. Mater. Chem. B, 2014, 2, 2793 RSC.
- X. Yuan, Z. Luo, Q. Zhang, X. Zhang, Y. Zheng, J. Y. Lee and J. Xie, ACS Nano, 2011, 5, 8800 CrossRef CAS PubMed.
- C. Wang, H. Cheng, Y. Sun, Q. Lin and C. Zhang, ChemNanoMat, 2015, 1, 27 CrossRef CAS.
- H. Cao, Z. Chen, H. Zheng and Y. Huang, Biosens. Bioelectron., 2014, 62, 189 CrossRef CAS PubMed.
- N. K. Das, S. Ghosh, A. Priya, S. Datta and S. Mukherjee, J. Phys. Chem. C, 2015, 119, 24657 CAS.
- C. Wang, Y. Yao and Q. Song, Colloids Surf., B, 2016, 140, 373 CrossRef CAS PubMed.
- Z. Wang, A. S. Susha, B. Chen, C. Reckmeier, O. Tomanec, R. Zboril, H. Zhong and A. L. Rogach, Nanoscale, 2016, 8, 7197 RSC.
- Y. Huang, Y. Lai, S. Shi, S. Hao, J. Wei and X. Chen, Chem.–Asian J., 2015, 10, 370 CrossRef CAS PubMed.
- J. Mou, P. Li, C. Liu, H. Xu, L. Song, J. Wang, K. Zhang, Y. Chen, J. Shi and H. Chen, Small, 2015, 11, 2275 CrossRef CAS PubMed.
- C. Wang and Y. Huang, Nano, 2013, 8, 1350054 CrossRef.
- Y. Xie, A. Riedinger, M. Prato, A. Casu, A. Genovese, P. Guardia, S. Sottini, C. Sangregorio, K. Miszta, S. Ghosh, T. Pellegrino and L. Manna, J. Am. Chem. Soc., 2013, 135, 17630 CrossRef CAS PubMed.
- M. Behboudnia and B. Khanbabaee, J. Cryst. Growth, 2007, 304, 158 CrossRef CAS.
- X. Jia, J. Li, L. Han, J. Ren, X. Yang and E. Wang, ACS Nano, 2012, 6, 3311 CrossRef CAS PubMed.
- M. W. Majewski, I. L. Bolotin and L. Hanley, ACS Appl. Mater. Interfaces, 2014, 6, 12901 Search PubMed.
- R. V. Goncalves, R. Wojcieszak, H. Wender, C. S. B. Dias, L. L. R. Vono, D. Eberhardt, S. R. Teixeira and L. M. Rossi, ACS Appl. Mater. Interfaces, 2015, 7, 7987 CAS.
- X. Lu, Y. Ishida, M. T. Nguyen and T. Yonezawa, J. Mater. Chem. C, 2015, 3, 8358 RSC.
- E. Vanecht, K. Binnemans, J. W. Seo, L. Stappers and J. Fransaer, Phys. Chem. Chem. Phys., 2011, 13, 13565 RSC.
- T. Torimoto, Y. Ohta, K. Enokida, D. Sugioka, T. Kameyama, T. Yamamoto, T. Shibayama, K. Yoshii, T. Tsuda and S. Kuwabata, J. Mater. Chem. A, 2015, 3, 6177 CAS.
- T. Sumi, S. Motono, Y. Ishida, N. Shirahata and T. Yonezawa, Langmuir, 2015, 31, 4323 CrossRef CAS PubMed.
- Y. Ishida, T. Sumi and T. Yonezawa, New J. Chem., 2015, 39, 5895 RSC.
- Y. Ishida, I. Akita, T. Sumi, M. Matsubara and T. Yonezawa, Sci. Rep., 2016, 6, 22928 CrossRef PubMed.
- K. Nakagawa, T. Narushima, S. Udagawa and T. Yonezawa, J. Phys.: Conf. Ser., 2013, 417, 012038 CrossRef.
- Y. Ishida, R. Nakabayashi, M. Matsubara and T. Yonezawa, New J. Chem., 2015, 39, 4227 RSC.
- T. Torimoto, K. Okazaki and T. Kiyama, Appl. Phys. Lett., 2006, 89, 243117 CrossRef.
- G. Liu, T. Schulmeyer, J. Broetz, A. Klein and W. Jaegermann, Thin Solid Films, 2003, 431–432, 477 CrossRef CAS.
- R. Wick and S. D. Tilley, J. Phys. Chem. C, 2015, 119, 26243 CAS.
- H. Wender, L. F. de Oliveira, A. F. Feil, E. Lissner, P. Migowski, M. R. Meneghetti, S. R. Teixeira and J. Dupont, Chem. Commun., 2010, 46, 7019 RSC.
- C. Vazquez-Vazquez, M. Banobre-Lopez, A. Mitra, M. A. Lopez-Quintela and J. Rivas, Langmuir, 2009, 25, 8208 CrossRef CAS PubMed.
- A. H. Pakiari and Z. Jamshidi, J. Phys. Chem. A, 2010, 114, 9212 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of preparation method and characterization; UV-vis spectra of PEG, mixture of MUA and PEG; XPS spectra of copper nanoclusters; 3D photoluminescence mapping for only MUA in PEG and for nanoclusters in a mixture of MUA and PEG; fraction of nanoclusters with size of 2 nm or less plotted versus MUA amount; photoluminescence spectra of the as-synthesized and stored nanoclusters' dispersion. See DOI: 10.1039/c6ra17291a |
| This journal is © The Royal Society of Chemistry 2016 |