A nanoporous photosensitizing hydrogel based on chitosan cross-linked by zinc phthalocyanine: an injectable and pH-stimuli responsive system for effective cancer therapy†
Ali Reza Karimi*a,
Azam Khodadadia and
Mahnaz Hadizadehb
aDepartment of Chemistry, Faculty of Science, Arak University, Arak, 38156-88349, Iran. E-mail: a-karimi@araku.ac.ir
bDepartment of Biotechnology, Iranian Research Organization for Science and Technology, Tehran 3353136846, Iran
First published on 19th September 2016
Abstract
Although zinc phthalocyanines (ZnPcs) have promising applications in photodynamic therapy (PDT), their therapeutic efficacy suffer from their low solubility in the biological environment and their lack of tumor selectivity. Herein, to achieve the best PDT efficacy of hydrophobic zinc phthalocyanine as a photodynamic agent, we report a facile approach to prepare a new pH-sensitive self-healable and injectable hydrogel conjugated tetra-aldehyde functionalized zinc phthalocyanine (TA-ZnPc) (6 wt% w/w) through a dynamic covalent Schiff-base linkage between benzaldehyde groups at TA-ZnPc β-ends and NH2 groups on chitosan as a safe carrier with a pH sensitive photosensitizer delivery system for localized cancer therapy. The molecular and geometric structure of TA-ZnPc also had notable effects in determining the hydrogel microstructure. The TA-ZnPc was employed not only as a photosensitizer agent for PDT but also as a cross-linking gelator for the preparation of a nanoporous hydrogel with high elasticity and unprecedentedly large surface area. The as-prepared pH-sensitive hydrogel can release TA-ZnPc in the acidic environment of tumors directly by evading the circulation system. TA-ZnPc was released from 1 to 8 days due to hydrolysis of the cross-linking linkage in acidic pH. The injectable hydrogel structure was characterized by FT-IR, 1H NMR, SEM and rheological measurements. Its dynamic nature imparts the self-healing capability of the network, as confirmed by the rheological recovery test, microscopic and macroscopic observations. Additionally, singlet-oxygen generation of the hydrogel conjugated TA-ZnPc could be finely controlled by varying pH which could manipulate the TA-ZnPc release behavior from the hydrogel. The photodynamic effect of hydrogel conjugated TA-ZnPc at different pH values was studied for two cell lines, namely (MDA-MB-231) and (A435). Conjugation of TA-ZnPc to a hydrogel can lead to much better viability under dark conditions than that of free TA-ZnPc at the same concentrations. The viability of cells incubated with the hydrogel was decreased significantly at acidic pH after laser irradiation.
Introduction
Photodynamic therapy (PDT) is an effective non-aggressive therapeutic modality against a variety of cancers, based on the tumor-localized generation of singlet oxygen by specific irradiation of photosensitizers (PSs).1,2 The excited photosensitizer in a triplet state reacts with molecular oxygen and forms cytotoxic reactive oxygen species (ROS), especially singlet oxygen (1O2) that destroys tumor cells and tissues.3–5 On the other hand, an ideal photosensitizer is non-toxic to the host in the absence of light in physiological conditions, accumulates preferentially in tumor tissue and should possess a high absorption coefficient, chemical stability, fast distribution and high quantum singlet oxygen generation yield.6–8 Among the most promising photosensitizer candidates for PDT, light-absorbing organic molecules such as zinc phthalocyanines (ZnPcs) attracted attention due to their intense absorption in the biological wavelength window (around 670 nm) with a greater penetration of tissue and their robust efficiency to produce singlet oxygen.9–11 However, most phthalocyanines (Pcs) are hydrophobic and may aggregate in a biological environment and form stacks through π–π aromatic interactions and thus significantly reduce their PDT efficacy.12–14 Even for hydrophilic Pcs, the efficiency of PDT is limited due to the low selectivity of the currently available photosensitizer for clinical uses, which causes undesirable side effects on nearby normal tissues.15,16 Therefore, selective localization of ZnPcs in tumors is critical for effective PDT. In order to overcome these defects, various carrier systems such as polymeric micelles, liposomes, nanoparticles and microspheres have been investigated to fabricate a stable dispersion of photosensitizers in aqueous systems.17–22 However, most of these Pc carriers are transported along the blood circulatory to tissues and penetrate from blood vessel walls into targeted tumor site.23 As a result, some challenges still persist, including reducing therapeutic efficacy due to poor loading of Pc, increasing normal tissue toxicity related to Pc payload leaking from the carriers into the body before reaching the target cells, time-consuming fabrication and encapsulation processes.24–26In this respect, an alternative approach to achieve the best localized tumor therapy is conjugation of ZnPc to injectable hydrogels. These hydrogels can be used as a promising carrier for biomedical applications due to their tunable properties, biocompatibility and minimal invasion because of their high resemblance with natural extracellular matrices.27–29 Chitosan (CS), a natural product-based biodegradable polymer, is a safe and effective drug carrier with bioadhesive properties and injectable in situ gelling formulations.30 These in situ hydrogels can be prepared by physical interactions (electrostatic or hydrophobic interactions) or through the formation of dynamic covalent bonds. However, the sustained and localized release in covalently cross-linked hydrogels can be easily performed in response to external stimuli such as changes in temperature or pH.31 Among the various cross-linking systems that can be utilized for dynamic covalent network, Schiff-base cross-linked injectable hydrogels have tunable gelation, biodegradability, and self-repair functions, which can release the PSs when triggered by unique biological stimuli such as pH.32 However, fabrication of chemically cross-linked hydrogels based on chitosan with superb self-healing behavior, high elasticity and significant response properties has become a challenging and fascinating topic.33 Self-healing injectable hydrogels based on chitosan can be applied through a syringe and undergo a sol–gel transition under shear conditions. They can be injected under shear stress because of their shear-thinning properties and quickly recover after removal of the shear stress.34 On the other hand, PSs can be mixed with precursor solutions and loaded at target tumor tissues by an in situ gelation right after the injection.35
Herein, we report the first successful use of hydrophobic tetra-aldehyde functionalized zinc phthalocyanine (TA-ZnPc) as a cross-linker for the preparation of nanoporous network based on chitosan hydrogel that is totally devoid of conventional cytotoxic small molecule cross-linkers. The properties of TA-ZnPc such as the structure, size and rigidity of molecule played important roles in determining microstructure and properties of the resulted hydrogels.36 Additionally, TA-ZnPc can be implemented in the design of self-healing network through dynamic covalent Schiff-base (also known as imine, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) linkage. As it is evidenced form its name, the self-healing hydrogel can repair itself automatically and in the case of Schiff base hydrogel this can happen through a dynamic imine formation. Because of its dynamic equilibrium, the Schiff-base linkage could be considered as a quasi-covalent linkage, and the cleavage and regeneration of the imine bond keep occurring in the hydrogel network, which invoke the self-healing concept. On the other hand, the in situ hydrogel was employed as an effective ZnPc carrier for PDT, which causes critical damage to cancer cells while avoiding harm to normal cells. The TA-ZnPc release is induced based on dynamic Schiff-base linkage in tumor tissues. In fact, imines are one of the acid-labile linkers most commonly used in drug delivery systems for cancer treatment. Due to the acidic tumor environment (pH 6.5–6.8) different from the normal tissues (7.4),37 pH-sensitive imines bonds are designed to remain stable at physiological pH, but degrade quickly in the mildly acidic environment of tumor tissues, leading to rapid ZnPc release. Therefore, injectable hydrogel can release TA-ZnPc in tumor directly by evading the circulation system. We first conjugated TA-ZnPc to chitosan via a Schiff base linkage between benzaldehyde groups at ZnPcTa β-ends and NH2 groups on chitosan. As depicted in Scheme 1, TA-ZnPc delivery from the injectable hydrogel can be triggered in response to pH change. The release of TA-ZnPc from the self-healable and injectable hydrogel was monitored upon pH changes and the cytotoxicites on human cancer cell lines MDA-MB-231 and A435 were evaluated.
CH–) linkage. As it is evidenced form its name, the self-healing hydrogel can repair itself automatically and in the case of Schiff base hydrogel this can happen through a dynamic imine formation. Because of its dynamic equilibrium, the Schiff-base linkage could be considered as a quasi-covalent linkage, and the cleavage and regeneration of the imine bond keep occurring in the hydrogel network, which invoke the self-healing concept. On the other hand, the in situ hydrogel was employed as an effective ZnPc carrier for PDT, which causes critical damage to cancer cells while avoiding harm to normal cells. The TA-ZnPc release is induced based on dynamic Schiff-base linkage in tumor tissues. In fact, imines are one of the acid-labile linkers most commonly used in drug delivery systems for cancer treatment. Due to the acidic tumor environment (pH 6.5–6.8) different from the normal tissues (7.4),37 pH-sensitive imines bonds are designed to remain stable at physiological pH, but degrade quickly in the mildly acidic environment of tumor tissues, leading to rapid ZnPc release. Therefore, injectable hydrogel can release TA-ZnPc in tumor directly by evading the circulation system. We first conjugated TA-ZnPc to chitosan via a Schiff base linkage between benzaldehyde groups at ZnPcTa β-ends and NH2 groups on chitosan. As depicted in Scheme 1, TA-ZnPc delivery from the injectable hydrogel can be triggered in response to pH change. The release of TA-ZnPc from the self-healable and injectable hydrogel was monitored upon pH changes and the cytotoxicites on human cancer cell lines MDA-MB-231 and A435 were evaluated.
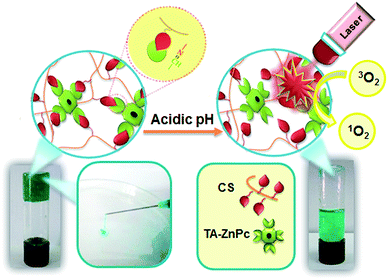 | ||
| Scheme 1 Schematic illustration of pH-sensitive and injectable covalently cross-linked hydrogel for in situ TA-ZnPc delivery for PDT. | ||
Results and discussion
Synthesis and characterization of TA-ZnPc/CS hydrogel
The target zinc phthalocyanine, peripherally substituted with benzaldehyde groups was successfully prepared under microwave irradiation at 300 W for 15 minutes through cyclotetramerization reaction of the corresponding phthalonitrile 3 (Scheme 2). The tetra-aldehyde functionalized zinc phthalocyanine was fully characterized by FT-IR, 1H NMR and MALDI-TOF-MS of CN band (see ESI†). Fig. S1† shows after cyclotetramerization of 4-(4-formylphenoxy)phthalonitrile (FPPht), the IR spectrum of phthalocyanine lacked the CN band, completely.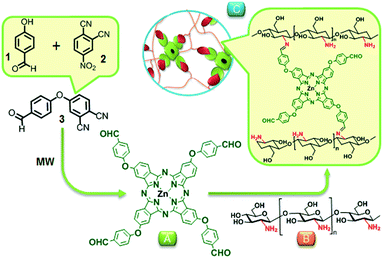 | ||
| Scheme 2 Chemical structure of in situ formation of TA-ZnPc conjugated chitosan. (A) TA-ZnPc; (B) CS; and (C) proposed structure of the TA-ZnPc/CS hydrogel through Schiff-based reaction. | ||
The MALDI-TOF mass spectrum of the TA-ZnPc gave the characteristic molecular ion peak at m/z: 1056 [M]+ by which the proposed structure was confirmed (Fig. S2, ESI†). Then, the injectable hydrogel covalently cross-linked with TA-ZnPc was synthesized through the Schiff's base reaction between amino groups on chitosan and benzaldehyde groups on TA-ZnPc termini. Briefly, after homogeneously mixing chitosan solution and TA-ZnPc at a concentration of 6 wt%, the TA-ZnPc conjugated chitosan (TA-ZnPc/CS) hydrogel was prepared at the end of mixing process (25 °C, <30 s). The formation of injectable hydrogel was further confirmed by FT-IR and 1H NMR. To confirm the preparation of covalently cross-linked hydrogel, we performed FT-IR analysis of TA-ZnPc, pure CS and TA-ZnPc/CS hydrogel as well as freeze-dried hydrogel (Fig. S3, ESI†). The absorption band specific to the symmetric vibration C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of TA-ZnPc at 1732 cm−1 fully disappeared in the spectrum of freeze-dried hydrogel, while a new peak of imine band C
O of TA-ZnPc at 1732 cm−1 fully disappeared in the spectrum of freeze-dried hydrogel, while a new peak of imine band C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N at 1639 cm−1 appeared on this curve, verifying the reaction between CS and tetra aldehyde groups on TA-ZnPc as cross-linker. In order to confirm the presence of zinc phthalocyanine in the modified CS hydrogel as a cross-linker through dynamic Schiff-base reaction, the 1H NMR spectra of TA-ZnPc and TA-ZnPc/CS hydrogel were obtained. In the 1H NMR spectrum of TA-ZnPc/CS hydrogel, disappearance of the CHO peak at 10.02 ppm and appearance of a new signal (8.05 ppm; –N
N at 1639 cm−1 appeared on this curve, verifying the reaction between CS and tetra aldehyde groups on TA-ZnPc as cross-linker. In order to confirm the presence of zinc phthalocyanine in the modified CS hydrogel as a cross-linker through dynamic Schiff-base reaction, the 1H NMR spectra of TA-ZnPc and TA-ZnPc/CS hydrogel were obtained. In the 1H NMR spectrum of TA-ZnPc/CS hydrogel, disappearance of the CHO peak at 10.02 ppm and appearance of a new signal (8.05 ppm; –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, imine) supports the formation of Schiff's cross-linkages between amine groups of CS and aldehyde groups on TA-ZnPc termini (Fig. S4, ESI†). Interestingly, the signals of TA-ZnPc that was insoluble in water were clearly observed, which indicated the TA-ZnPc had been successfully attached onto chitosan backbone via the covalent interaction. In addition, although the aggregation behavior of phthalocyanine in polar organic solvents at the concentrations used for the NMR measurements may lead to broadening of the aromatic signals,38 the TA-ZnPc conjugated chitosan gave a well-resolved spectrum with sharp peaks with no broadening in aromatic region at 7.78 ppm implying that the aggregation behavior is totally absent in water.
CH–, imine) supports the formation of Schiff's cross-linkages between amine groups of CS and aldehyde groups on TA-ZnPc termini (Fig. S4, ESI†). Interestingly, the signals of TA-ZnPc that was insoluble in water were clearly observed, which indicated the TA-ZnPc had been successfully attached onto chitosan backbone via the covalent interaction. In addition, although the aggregation behavior of phthalocyanine in polar organic solvents at the concentrations used for the NMR measurements may lead to broadening of the aromatic signals,38 the TA-ZnPc conjugated chitosan gave a well-resolved spectrum with sharp peaks with no broadening in aromatic region at 7.78 ppm implying that the aggregation behavior is totally absent in water.
Morphology of TA-ZnPc/CS hydrogel
The internal morphology of the TA-ZnPc/CS hydrogel is investigated by scanning electron microscopy (SEM), as shown in Fig. 1. The obtained hydrogel is freeze-dried, which maintains its high surface area and porous nanostructure. The hydrogel displayed nanoporous cross-linked network with uniform diameters ranging from 20 to 50 nm.Rheological characterization of TA-ZnPc conjugated chitosan hydrogel
The viscoelastic properties of TA-ZnPc/CS hydrogel at different pH were evaluated by monitoring the variations of storage modulus (G′) and loss modulus (G′′) as a function of frequency (ω) at a fixed strain, γ = 1.0%. As shown in Fig. 2A, the formation of covalently cross-linked hydrogel is revealed by the wide linear viscoelastic region in the dynamic frequency sweep experiments and further confirmed by the fact that the G′ value is higher than the corresponding G′′ value. Nevertheless, an apparent decrease of ratio of storage/loss moduli was observed under acidic conditions. Notably, it could have resulted from pH-sensitive properties of the TA-ZnPc/CS hydrogel.Self-healing properties of TA-ZnPc/CS hydrogel
In particular, for an injectable hydrogel as a qualified photosensitizer carrier, it is important that the hydrogel could rapidly self-heal and restore to its original gel state after inflicted damage. Therefore, rheology analyses of hydrogel were carried out to monitor qualitatively the self-healing process (Fig. 2B). First, a hydrogel was prepared as described above tested for the measurement of G′ and G′′. The storage modulus was much greater than the loss modulus over the whole range of frequency, demonstrating that a hydrogel is formed. Then, the hydrogel was subsequently cut into pieces. After cutting the hydrogel, the broken hydrogel exhibited lower G′ than the original hydrogel. However, the storage modulus value increased with time and finally achieved a similar value to that of the original hydrogel. Moreover, the G′ and G′′ values of the self-healed hydrogel versus frequency were approximately the same as those of the original hydrogel, indicating the self-healing property of hydrogel. In addition, rheology test was performed to measure the elastic response of the self-healing hydrogel through strain amplitude sweep (Fig. 2C). The G′ value decreased rapidly above the critical strain region (γ = 80%). From the results, the G′ and the loss modulus G′′ curve intersect at the strain of 80%, indicating that the state of gel is between solid and fluid near this critical point. With the increasing of the strain to 800%, the G′ intensely decreased due to the collapse of the gel network. Additionally, the continuous step strain measurements were carried out to test the rheology recovery behavior of the hydrogel. As shown in Fig. 2D the G′ value decreased from 900 to 7 Pa under a high amplitude force (γ = 80%, frequency = 1 Hz, kept for few minutes), confirmed by a loose network. However, when the amplitude was decreased to strain (γ = 1%) at the same frequency (1.0 Hz), the G′ quickly recovered to the initial value and the gel returned to the original state, indicating the recovery of the cross-linked network.Similarly, when the larger strains (300% and 800%) and small strain (1.0%) were alternatively applied later, the G′ also quickly restored the initial value. This self-recovery process was continued for 3 cycles under varying strain to establish the self-healing property.
Furthermore, we demonstrated the macroscopic self-healing behavior of the TA-ZnPc/CS hydrogel by cutting the hydrogel into two pieces followed by connecting them back to the original shape (Fig. S5A, ESI†). Moreover, we monitored the self-healing process of the prepared hydrogel by using an optical microscope. Optical microscopic images clearly reveal the two hydrogel fragments could adhere to each other instantly when brought into contact and automatically heal into one integral piece at room temperature (Fig. S5B, ESI†). The crack (width 1 mm) made by a razor blade completely disappeared after 15 minutes and there was no discernable difference between the healed and the undamaged area.
UV-visible absorption spectra
To evaluate the optical properties of TA-ZnPc and TA-ZnPc/CS hydrogel, the UV-vis spectra were investigated. The spectroscopic properties of TA-ZnPc and TA-ZnPc conjugated chitosan are summarized in Table 1. Absorption spectra of TA-ZnPc in DMF and TA-ZnPc/CS hydrogel in DMF and H2O showed very similar absorption spectra with a sharp Q-band at the red visible region (670–685 nm) (Fig. 3A). This indicates that the photophysical properties of the TA-ZnPc did not change obviously after conjugation with chitosan. Furthermore, in response to the decreasing pH, TA-ZnPc conjugated chitosan showed significantly increased absorption intensity (Fig. S6, ESI†), corresponding to pH-sensitive properties of the Schiff-base cross-linked hydrogel. This result verified that the hydrogel destructed under acidic environment and leading to rapid TA-ZnPc release from the TA-ZnPc/CS hydrogel due to the breakage of the pH cleavable Schiff's base bonds.| Compounds | Solvent | λmax (nm) | (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) ε) |
|---|---|---|---|
| TA-ZnPc | DMF | 670 | 5.36 |
| TA-ZnPc/CS | DMF | 675 | 5.25 |
| TA-ZnPc/CS | H2O | 685 | 5.22 |
Singlet oxygen quantum yield
Singlet oxygen production from the TA-ZnPc/CS hydrogel was detected to assess the potential PDT effect in vitro. The production efficiency of 1O2 induced by TA-ZnPc/CS hydrogel under irradiation could be evaluated with the singlet oxygen quantum yields (ΦΔ), which was determined by a steady-state method using 1,3 diphenylisobenzofuran (DPBF) as the 1O2 indicator and ZnPc as the standard. As shown in Fig. 3B, the absorption of DPBF displayed a continuous decrease at 417 nm within 25 min when solution of TA-ZnPc/CS hydrogel was excited at 660 nm. Fig. 3C clearly showed that the photo-degradation degree of DPBF with TA-ZnPc/CS hydrogel was significantly dependent on solution pH. DPBF degradation against irradiation time (25 min) was monitored and the linear [DPBF]-time relationship was observed for the DPBF degradation at pH 7.4 and pH 5.0 (Fig. S7, ESI†).The singlet oxygen quantum yield for TA-ZnPc/CS hydrogel (0.49) at pH 5.0 present a slight decrease compared with ZnPc in DMF (0.56),39 showing the efficient photodynamic activity of TA-ZnPc conjugated chitosan in the acidic environment. We also investigated the singlet oxygen quantum yield for TA-CuPc/CS and TA-NiPc/CS hydrogels in DMF (Table S1, ESI†). From the results, the presence of the inserted central metal ion in phthalocyanine strongly influence its singlet oxygen quantum yield.40 Acceptable value of the quantum yield for effective PDT is for TA-ZnPc/CS hydrogel, while the hydrogels containing phthalocyanines with Cu and Ni metals in the central ring have very small quantum yields in generating singlet oxygen.
In vitro TA-ZnPc release from TA-ZnPc/CS hydrogel
In vitro TA-ZnPc release behavior from the TA-ZnPc/CS hydrogel was investigated at pH 5.0, 6.8 and 7.4. The TA-ZnPc content can be calculated according to the standard concentration–absorbency curve of TA-ZnPc (Fig. S8, ESI†). The release of TA-ZnPc from hydrogel at different pH is plotted as cumulative release versus time in Fig. 3D. The release of TA-ZnPc was extinguished at pH 7.4, and only about 30% of TA-ZnPc was released from the hydrogel within 8 days. However, the rate of TA-ZnPc release under acidic conditions (pH 6.8 and 5.0) was much higher than at a physiological pH. For example, about 55% of TA-ZnPc was released from the hydrogel at pH 6.8 in 8 days. Even higher release of TA-ZnPc observed at pH 5.0 around 85% within 8 days. This pH-activatable behavior may be attributed to the acid-labile imine linkage of the TA-ZnPc conjugated chitosan, which is stable at physiological pH and labile at low pH.pH-responsive test
pH-responses of TA-ZnPc/CS hydrogel was tested and shown in Fig. 4. First, the aqueous solution of HCl (20 μL, 6 M) was added to the hydrogel, and the hydrogel was dissolved in ∼5 min with vortex. Then, the aqueous solution of NaOH (20 μL, 6 M) was added to the hydrogel. Subsequent addition of NaOH solution, neutralized acid, and the TA-ZnPc/CS hydrogel was regenerated in ∼30 s. This process could be repeated 4 times in our experiments.In vitro studies on human cancer cell lines MDA-MB-231 and A435
The superb results from photophysical properties and release behavior prompted us to introduce TA-ZnPc/CS hydrogel into in vitro tests on human cancer cell lines MDA-MB-231 and A435 for toxicity and photodynamic behavior.In order to evaluate the therapeutic effect of TA-ZnPc/CS hydrogel, cell viabilities with different treatments were measured. We first measured the dark toxicity of TA-ZnPc and TA-ZnPc conjugated chitosan via a standard MTT assay in vitro, using MDA-MB-231 (human breast cancer cells) and A435 (human melanoma cells). Different amounts of TA-ZnPc and TA-ZnPc conjugated chitosan were added to cells that had incubated for 24 h. After 24 h of incubation, the cell viability was measured via MTT assay. As can be seen in Fig. 5, the cell viabilities of MDA-MB-231 and A435 were >97% and >98%, respectively, even at the high concentration of hydrogel (0.2 μg mL−1), compared to free TA-ZnPc, which showed viabilities lower than 90%. Furthermore, the results indicating that hydrogels have good biocompatibility and low cytotoxicity.
Next, we tested the in vitro cytotoxicity and the PDT effect of the TA-ZnPc/CS hydrogel, where cells were incubated in a culture medium containing a series of concentrations of TA-ZnPc conjugated chitosan for 24 h, and then irradiated with a 660 nm laser at a power density of 5 J cm−2 for 25 min. The quantitative phototoxicities of various concentrations of TA-ZnPc conjugated chitosan are shown in Fig. 6. It is clearly seen that after laser irradiation, for each cancer cell line, viability decreased with increasing concentrations of the TA-ZnPc/CS hydrogel. Interestingly, the viability of the cells falls sharply for the TA-ZnPc/CS hydrogel at pH 5.0. As seen in Fig. 6, the best PDT behavior was demonstrated with the dosage of 0.2 μg mL−1 of TA-ZnPc/CS hydrogel at pH 5.0.
Moreover, in order to visualize the photodynamic activity of the as-prepared hydrogel, the morphology of the MDA-MB-231 cells treated with TA-ZnPc conjugated hydrogel, without irradiation and under irradiation was studied under an inverted light microscope.
As shown in Fig. 7, there is morphological difference between cells treated with photosensitizer after irradiation at pH 5.0.
 | ||
| Fig. 7 Microscopy image (40×) of MDA-MB-231 cells treated with 0.2 μg mL−1 of TA-ZnPc/CS hydrogel at pH 5.0 (A) without irradiation, and (B) under irradiation (25 min irradiation). | ||
Experimental
Materials and instrumentations
All chemicals were purchased from Sigma-Aldrich and were used without further purification. Fourier transform infrared (FT-IR) data of FPPht, TA-ZnPc and TA-ZnPc/CS hydrogel were recorded on a Unicom Galaxy Series FTIR 5000 Spectrophotometer. Each spectrum was recorded over the region 4000–400 cm−1. 1H NMR measurements of FPPht, TA-ZnPc and TA-ZnPc/CS hydrogel were determined on a Bruker Avance 300 MHz spectrometer. DMSO-d6 and D2O were used as the solvents and the solvent signal was used for internal calibration (DMSO-d6: δ (1H) = 2.5 ppm), (D2O: δ (1H) = 4.79 ppm). MALDI-TOF-MS spectrum of TA-ZnPc was performed on Agilent 6220 Accurate-Mass (Santa Clara California, USA). Electronic spectral measurements of TA-ZnPc and CS/TA-ZnPc hydrogel were carried out using Perkin-Elmer Lamda double beem spectrophotometer in the range 190–900 nm. The surface morphology of CS/TA-ZnPc hydrogel was obtained using Field Emission Scanning Electron Microscope (Mira 3-XMU) after sputter coating with a thin layer of gold (Au) under vacuum. The rheological experiments performed on a rheometer (MCR 300). All the experiments were carried out at room temperature using 40 mm parallel plate with plate gap of 1.0 mm.Synthesis of tetra-aldehyde functionalized zinc phthalocyanine (TA-ZnPc)
The zinc phthalocyanine tetra-aldehyde (TA-ZnPc) used in this study was synthesized in two steps. First, a mixture of 4-hydroxybenzaldehyde 1 (0.122 g, 1 mmol), 4-nitrophthalonitrile 2 (0.173 g, 1 mmol), and anhydrous K2CO3 (0.138 g, 1 mmol) was dissolved in 2 mL DMF. Then, the resulting mixture was stirred at room temperature for 1 day. After that, 5 mL acetone and 4 mL water was added alternately to the reaction mixture to give 4-(4-formylphenoxy)phthalonitrile (FPPht) 3.41 The precipitate was washed with 5 mL hot water. In the next step, TA-ZnPc as a cross-linker and promising photosensitizer prepared by cyclization of compound 3 (0.10 g, 0.42 mmol) with anhydrous metal salt Zn(CH3COO)2 (0.03 g, 0.16 mmol) in the presence of a few drops of DBU in 2 mL dimethylaminoethanol (DMAE) under microwave irradiation at 300 W for 15 minutes. The resulting mixture was then cooled to room temperature. After that, 2 mL ethanol was added and the product was filtered under reduced pressure. Finally, the green solid was washed several times with hot ethanol.Preparation of TA-ZnPc conjugated chitosan (TA-ZnPc/CS) hydrogel
In a typical synthesis, a 3% (w/v) chitosan solution was prepared by dissolving certain amount of chitosan (0.1 g, medium viscosity, 75–80% deacetylated; Aldrich, St Louis, MO, USA) in 1% aqueous acetic acid. Then, a solution of cross-linker was prepared by dissolving 0.006 g TA-ZnPc in 2 mL aqueous DMF. The as-prepared TA-ZnPc solution was added to chitosan solution and the mixture was shaken with vortex, and gelation happened within 30 s. Then DMF was removed under reduced pressure and the hydrogels were rinsed with excess distilled water and ethanol.Hydrogel morphology
The morphology and porosity of the freeze-dried TA-ZnPc/CS hydrogel was examined with a scanning electron microscope (SEM, Mira 3-XMU, TESCAN). The cross-section morphologie was viewed using a SEM operated at an accelerating voltage of 15 kV. The hydrogel was frozen in liquid nitrogen and then freeze-dried for 4 days. Then, the freeze-dried hydrogel was cut into thin sections using a sharp blade, followed by sputter coating with gold.Rheological analyse
To assess the viscoelastic properties of hydrogel, we performed the rheological experiments on a rheometer (MCR 300). All the experiments were carried out at 37 °C using a 40 mm parallel plate with plate gap of 1.0 mm. The hydrogel was placed between the parallel plate and the platform with special care to avoid evaporation of water. The storage modulus (G′) and loss modulus (G′′) were measured as a function of frequency (ω) at a fixed strain, γ = 1.0%.Self-healing tests
The rheology tests of hydrogel was carried out to monitor qualitatively the self-healing process at 37 °C with a 40 mm-diameter parallel plate. Moreover, elastic response of the hydrogel was analysed through strain amplitude sweep. Based on the strain amplitude sweep results, the continuous step strain measurement was carried out to test the rheology recovery behavior of the hydrogel. Moreover, we indicated the self-healing behavior of the TA-ZnPc/CS hydrogel by microscopic and macroscopic observations.In vitro TA-ZnPc release behavior
To investigate the TA-ZnPc release behavior from the hydrogel, the TA-ZnPc conjugated chitosan prepared was transferred into a dialysis bag (MW cut-off 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and was incubated in 20 mL 0.01 M PBS at pH 7.4, 6.8 and 5.0 and 37 °C in a water bath under mild agitation with shaking rate of 80 rpm. The supernatant was collected at predetermined time points (ranging from 1 day to 8 days) and replaced with an equal volume of fresh PBS. The released amount of TA-ZnPc was measured by the absorbance at 685 nm with the help of a calibration curve of TA-ZnPc in the same buffer. Then, the relative percentage of the released TA-ZnPc in different pH were calculated as a function of time.
000) and was incubated in 20 mL 0.01 M PBS at pH 7.4, 6.8 and 5.0 and 37 °C in a water bath under mild agitation with shaking rate of 80 rpm. The supernatant was collected at predetermined time points (ranging from 1 day to 8 days) and replaced with an equal volume of fresh PBS. The released amount of TA-ZnPc was measured by the absorbance at 685 nm with the help of a calibration curve of TA-ZnPc in the same buffer. Then, the relative percentage of the released TA-ZnPc in different pH were calculated as a function of time.
ROSs generation ability of TA-ZnPc/CS hydrogel
The generation of singlet oxygen for the TA-ZnPc conjugated chitosan (c = 20 μM) as a photosensitizer was detected chemically using 1,3-diphenylisobenzofuran (DPBF) (100 μM) as a singlet oxygen chemical quencher in DMF with ZnPc as reference using eqn (1):42
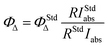 | (1) |
Cytotoxicity assay
The in vitro cytotoxicity of various concentrations of TA-ZnPc and TA-ZnPc conjugated chitosan were evaluated against human cancer cell lines MDA-MB-231 and A435 by methyl thiazolyl tetrazolium (MTT) assay. All the tests were conducted three times. MDA-MB-231 (human breast cancer cells) and A435 (human melanoma cells) were obtained from the Institute of Pasture, Tehran, Iran. Cells (8 × 103 cells per well) were seeded in 96 well cell culture plates and then maintained in culture medium under an atmosphere of air containing 5% CO2 in an incubator. After 24 h incubation, the culture medium was removed and the cells were washed with phosphate buffered saline (PBS). Then, 100 μL of PBS containing different concentrations of TA-ZnPc or TA-ZnPc/CS hydrogel was added to in corresponding wells and co-incubated for another 24 h without light interference. The medium was replaced with 100 mL of MTT solution and further cultured for 4 h. The absorbance of the medium was measured using a microplate reader. Viability of cells treated with different various concentrations of TA-ZnPc and TA-ZnPc was compared with untreated controls.In vitro photodynamic assay43
To study the PDT efficiency of TA-ZnPc conjugated chitosan, cells (8 × 103 cells per well) were seeded in 96 well cell culture plates and incubated for 24 h (37 C, 5% CO2). The culture medium was then replaced with fresh medium (pH 7.4 or 5.0) for 4 h. After that, solutions containing different concentrations of TA-ZnPc/CS hydrogel were added to in corresponding wells and incubated for 24 h. After a further incubation of 24 h, cells were washed and covered with fresh medium. Then the cells treated with TA-ZnPc conjugated chitosan were irradiated by a 660 nm laser (5 j cm−2, 25 min). After irradiation, the in vitro photodynamic effects were assessed by cell viability according to the standard MTT assay as mentioned above. The percentage of phototoxicity was calculated relatively to control cells.Conclusions
In summary, we have demonstrated the design and synthesis of photosensitizing hydrogel for effective cancer therapy. More interestingly, through interaction between the tetra-aldehyde functionalized zinc phthalocyanine and CS chain, Schiff-base linkage act to align the CS chains to form self-healing nanoporous structure with both rapid response properties and high elasticity. The cytotoxicity assay has proved low toxicity and the excellent biocompatibility for TA-ZnPc cross-linked with chitosan. TA-ZnPc release under acidic tumor environment, which promotes PDT to become a safer method for cancer therapy. Therefore, the injectable hydrogel conjugated with TA-ZnPc can act as a pH sensitive photosensitizer delivery system to achieve localized cancer treatment.Acknowledgements
The authors gratefully acknowledge from the Research Council of Arak University (Project No. 94.7610) for financial support of this work.Notes and references
- H. Park and K. Na, Biomaterials, 2013, 34, 6992–7000 CrossRef CAS PubMed
.
- A. P. Castano, P. Mroz and M. R. Hamblin, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef CAS PubMed
.
- C.-C. Chang, M.-C. Hsieh, J.-C. Lin and T.-C. Chang, Biomaterials, 2012, 33, 897–906 CrossRef CAS PubMed
.
- D. E. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed
.
- H. Wang, Z. Liu, S. Wang, C. Dong, X. Gong, P. Zhao and J. Chang, ACS Appl. Mater. Interfaces, 2014, 6, 3219–3225 CAS
.
- R. Liang, L. Ma, L. Zhang, C. Li, W. Liu, M. Wei, D. Yan, D. G. Evans and X. Duan, Chem. Commun., 2014, 50, 14983–14986 RSC
.
- U. Basu, I. Khan, A. Hussain, P. Kondaiah and A. R. Chakravarty, Angew. Chem., Int. Ed., 2012, 51, 2658–2661 CrossRef CAS PubMed
.
- G. Obaid, I. Chambrier, M. J. Cook and D. A. Russell, Angew. Chem., Int. Ed., 2012, 51, 6158–6162 CrossRef CAS PubMed
.
- S. Makhseed, M. Machacek, W. Alfadly, A. Tuhl, M. Vinodh, T. Simunek, V. Novakova, P. Kubat, E. Rudolf and P. Zimcik, Chem. Commun., 2013, 49, 11149–11151 RSC
.
- T. Nyokong, Coord. Chem. Rev., 2007, 251, 1707–1722 CrossRef CAS
.
- I. Gürol, M. Durmuş, V. Ahsen and T. Nyokong, Dalton Trans., 2007, 3782–3791 RSC
.
- F. Dumoulin, M. Durmuş, V. Ahsen and T. Nyokong, Coord. Chem. Rev., 2010, 254, 2792–2847 CrossRef CAS
.
- N. Sekkat, H. v. d. Bergh, T. Nyokong and N. Lange, Molecules, 2011, 17, 98–144 CrossRef PubMed
.
- Y. Li, J. Wang, X. Zhang, W. Guo, F. Li, M. Yu, X. Kong, W. Wu and Z. Hong, Org. Biomol. Chem., 2015, 13, 7681–7694 CAS
.
- M. Verbrugghe, S. Verhaeghe, K. Lauwaert, D. Beeckman and A. Van Hecke, Cancer Treat. Rev., 2013, 39, 610–621 CrossRef CAS PubMed
.
- Y. N. Konan, R. Gurny and E. Allémann, J. Mater. Chem. B, 2002, 66, 89–106 CAS
.
- Y. Chen, L. Rui, L. Liu and W. Zhang, Polym. Chem., 2016, 7, 3268–3276 RSC
.
- A. M. Master, M. E. Rodriguez, M. E. Kenney, N. L. Oleinick and A. S. Gupta, J. Pharm. Sci., 2010, 99, 2386–2398 CrossRef CAS PubMed
.
- N. Nombona, K. Maduray, E. Antunes, A. Karsten and T. Nyokong, J. Photochem. Photobiol., B, 2012, 107, 35–44 CrossRef CAS PubMed
.
- B.-P. Jiang, L.-F. Hu, X.-C. Shen, S.-C. Ji, Z. Shi, C.-J. Liu, L. Zhang and H. Liang, ACS Appl. Mater. Interfaces, 2014, 6, 18008–18017 CAS
.
- C. Liu, J. Guo, W. Yang, J. Hu, C. Wang and S. Fu, J. Mater. Chem., 2009, 19, 4764–4770 RSC
.
- F. B. Barlas, B. Demir, E. Guler, A. M. Senisik, H. A. Arican, P. Unak and S. Timur, RSC Adv., 2016, 6, 30217–30225 RSC
.
- J. B. Wolinsky, Y. L. Colson and M. W. Grinstaff, J. Controlled Release, 2012, 159, 14–26 CrossRef CAS PubMed
.
- T. J. Merkel, S. W. Jones, K. P. Herlihy, F. R. Kersey, A. R. Shields, M. Napier, J. C. Luft, H. Wu, W. C. Zamboni and A. Z. Wang, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 586–591 CrossRef CAS PubMed
.
- C. Lu, M. M. Xing and W. Zhong, Nanomedicine: Nanotechnology, Biology and Medicine, 2011, 7, 80–87 CrossRef CAS PubMed
.
- M. Ferrari, Nat. Rev. Cancer, 2005, 5, 161–171 CrossRef CAS PubMed
.
- H. Du, G. Zha, L. Gao, H. Wang, X. Li, Z. Shen and W. Zhu, Polym. Chem., 2014, 5, 4002–4008 RSC
.
- D. Das and S. Pal, RSC Adv., 2015, 5, 25014–25050 RSC
.
- J.-K. Cho, K.-Y. Hong, J. W. Park, H.-K. Yang and S.-C. Song, J. Drug Targeting, 2010, 19, 270–280 CrossRef PubMed
.
- H. T. Ta, C. R. Dass and D. E. Dunstan, J. Controlled Release, 2008, 126, 205–216 CrossRef CAS PubMed
.
- S. Aryal, C.-M. J. Hu and L. Zhang, ACS Nano, 2009, 4, 251–258 CrossRef PubMed
.
- J. Shi, W. Guobao, H. Chen, W. Zhong, X. Qiu and M. M. Xing, Polym. Chem., 2014, 5, 6180–6189 RSC
.
- X. Hao, H. Liu, Y. Xie, C. Fang and H. Yang, Colloid Polym. Sci., 2013, 291, 1749–1758 CAS
.
- M. Guvendiren, H. D. Lu and J. A. Burdick, Soft Matter, 2012, 8, 260–272 RSC
.
- Y. Wang, Q. Luo, R. Sun, G. Zha, X. Li, Z. Shen and W. Zhu, J. Mater. Chem. B, 2014, 2, 7612–7619 RSC
.
- N. B. McKeown, S. Makhseed and P. M. Budd, Chem. Commun., 2002, 2780–2781 RSC
.
- S. Ganta, H. Devalapally, A. Shahiwala and M. Amiji, J. Controlled Release, 2008, 126, 187–204 CrossRef CAS PubMed
.
- S. Gaspard and P. Maillard, Tetrahedron, 1987, 43, 1083–1090 CrossRef CAS
.
- S. G. Awuah, J. Polreis, V. Biradar and Y. You, Org. Lett., 2011, 13, 3884–3887 CrossRef CAS PubMed
.
- M. Çamur, M. Durmuş and M. Bulut, J. Photochem. Photobiol., A, 2011, 222, 266–275 CrossRef
.
- A. R. Karimi and A. Khodadadi, Tetrahedron Lett., 2012, 53, 5223–5226 CrossRef CAS
.
- A. Erdoğmuş and T. Nyokong, Dyes Pigm., 2010, 86, 174–181 CrossRef
.
- Y. Wang, B. Han, R. Shi, L. Pan, H. Zhang, Y. Shen, C. Li, F. Huang and A. Xie, J. Mater. Chem. B, 2013, 1, 6411–6417 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: FT-IR and 1H NMR of FPPht, TA-ZnPc, TA-ZnPc/CS, MALDI-TOF of TA-ZnPc, macroscopic and microscopic self-healing behaviour, optical properties of TA-ZnPc/CS at different pH, linear plot of absorbance of DPBF at pH 5.0 and 7.4, Standard curves of TA-ZnPc release at different pH. See DOI: 10.1039/c6ra17064a |
| This journal is © The Royal Society of Chemistry 2016 |






