Promising biomass-derived activated carbon and gold nanoparticle nanocomposites as a novel electrode material for electrochemical detection of rutin
Abstract
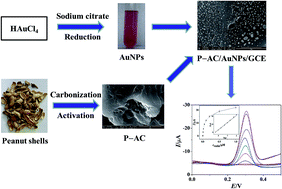
In this paper, six types of typical bio-wastes are used to prepare activated carbons (ACs) by high-temperature carbonization and activation with KOH. A novel electrochemical sensor for rutin was developed based on a peanut shell-derived activated carbon and gold nanoparticle composite modified glassy carbon electrode (P-AC/AuNPs/GCE). The as-synthesized ACs and composites were characterized by a variety of physicochemical techniques. The proposed sensor exhibits ideal electrochemical behavior for rutin with a wide linear range, low detection limit, and good selectivity. The desirable electrochemical performance enables the biomass-derived ACs and their composites to act as new sources of carbonaceous materials for electrochemical sensors.


 Please wait while we load your content...
Please wait while we load your content...