Synthesis and solid-state fluorescence of aryl substituted 2-halogenocinchomeronic dinitriles†
Abstract
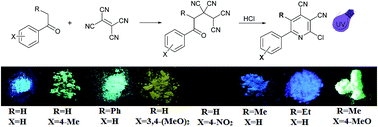
Based on the reaction of available adducts of tetracyanoethylene (TCNE) and aromatic ketones (4-aryl-4-oxoalkane-1,1,2,2-tetracarbonitriles) with hydrogen chloride, several aryl substituted 2-halogencinchomeronic dinitriles were synthesized. We found that aryl substituted derivatives, in contrast to alkyl substituted ones, possess an intensive solid state fluorescence. The relationship between the chemical structures and photophysical properties of these compounds was investigated.

 Please wait while we load your content...
Please wait while we load your content...