Effect of co-doping of alkali metal ions on Ca0.5RE1−x(MoO4)2:xEu3+ (RE = Y, La) phosphors with enhanced luminescence properties
Abstract
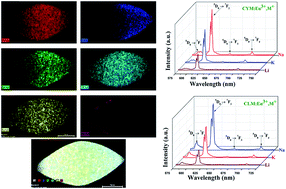
A series of Ca0.5RE1−x(MoO4)2:xEu3+,M+ (RE = Y, La; M = Li, K and Na) phosphors were synthesized by a facile hydrothermal method. X-ray diffraction suggested that these phosphors could be indexed to a scheelite tetragonal Ca0.5RE1−x(MoO4)2 (RE = Y, La) phase with a space group of I41/a with high purity. FESEM indicated the signs of bi-pyramid-like micro-architectures. Elemental mapping analysis provided evidence of the incorporation and uniform distribution of all the elements. X-ray photoelectron spectroscopy (XPS) confirmed the presence of all the elements. The PL properties of Ca0.5RE1−x(MoO4)2:xEu3+,M+ (RE = Y, La; M = Li, K and Na) phosphors were analysed extensively. The obtained results suggest that the characteristic red emission is due to the hypersensitive electric dipole 5D0 → 7F2 transition. The emission intensity of Ca0.5RE1−x(MoO4)2:xEu3+,Na+ (RE = Y, La) is higher than Li+ and K+ co-doped Ca0.5RE1−x(MoO4)2:xEu3+ (RE = Y, La). This can be explained by the charge compensation effect. Judd–Ofelt theory was adopted to examine the photophysical properties. The luminescence decay time values were estimated. The CIE chromaticity co-ordinates were derived from the emission spectra and the values were found to be close to the NTSC standard values. Hence, the prepared phosphors are suitable for display applications.


 Please wait while we load your content...
Please wait while we load your content...