Nitrogen- and oxygen-containing micro–mesoporous carbon microspheres derived from m-aminophenol formaldehyde resin for supercapacitors with high rate performance†
Siping Liua,
Xiujuan Chenab,
Xiaoli Lic,
Pengfei Huoa,
Yiqun Wanga,
Long Baia,
Wen Zhanga,
Maocheng Niua and
Zhiguo Li*a
aKey Laboratory of Bio-based Material Science and Technology, Ministry of Education, College of Materials Science and Engineering, Northeast Forestry University, Harbin, Heilongjiang 150040, China. E-mail: lizgmse@nefu.edu.cn
bBenjamin M. Statler College of Engineering and Mineral Resources, West Virginia University, Morgantown, WV 26506, USA
cHeilongjiang Key Laboratory of Molecular Design and Preparation of Flame Retarded Materials, College of Science, Northeast Forestry University, Harbin, Heilongjiang 150040, China
First published on 13th September 2016
Abstract
Nitrogen- and oxygen-containing micro-mesoporous carbon microspheres (NO-MMCMs) were prepared by a self-assembly hydrothermal synthesis in the presence of m-aminophenol as a carbon and nitrogen co-precursor and block copolymer F127 as a soft template, followed by carbonization and activation with KOH. The NO-MMCMs possess a high specific surface area (up to 3203 m2 g−1), large pore volume with a well-balanced micro- and mesoporosity (up to 1.93 cm3 g−1), moderate heteroatom content (6.64 at%) and micrometer-sized sphere-like morphology. Moreover, the heteroatom contents and porosity parameters (e.g. surface area and pore volume) can be easily controlled by the carbonization temperature in the range of 600–850 °C. When tested as a supercapacitor electrode in a three-electrode system using 6 M KOH solution as the electrolyte, the typical NO-MMCMs (NO-MMCMs-600) displays a high specific capacitance (309 F g−1 at 0.1 A g−1), excellent rate capability (229 F g−1 at 20 A g−1 with capacitance retention of 74%) and outstanding cycling stability (95% capacity retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles at 10 A g−1). Furthermore, high performance of reversible specific capacitance of 200 F g−1 at 20 A g−1 with high rate capability of 77% can be achieved in a two-electrode cell. The superior capacitive performances may be mainly because of both the spherical morphology and the high-surface-area hierarchical microporous/mesoporous structure, which allow the rapid transport of electrolyte ions through the carbon matrix, as well as the excellent synergistic effect of co-doping of N and O. These encouraging results demonstrate that the NO-MMCMs electrode materials with high current charge and discharge capability hold great potential for supercapacitor applications, where a fast charge/discharge is required.
000 charge/discharge cycles at 10 A g−1). Furthermore, high performance of reversible specific capacitance of 200 F g−1 at 20 A g−1 with high rate capability of 77% can be achieved in a two-electrode cell. The superior capacitive performances may be mainly because of both the spherical morphology and the high-surface-area hierarchical microporous/mesoporous structure, which allow the rapid transport of electrolyte ions through the carbon matrix, as well as the excellent synergistic effect of co-doping of N and O. These encouraging results demonstrate that the NO-MMCMs electrode materials with high current charge and discharge capability hold great potential for supercapacitor applications, where a fast charge/discharge is required.
Introduction
With the ever-increasing depletion of fossil fuel and the emergence of global warming problems, the utilization of renewable and clean energy sources like solar energy and wind energy catches people's universal attention. As such, there is a pressing need for low-cost, environmentally-friendly and advanced devices associated with clean and renewable energy storage and conversion. Representing a class of energy storage devices, supercapacitors, which can provide higher energy density as compared to conventional capacitors and deliver higher power capability as well as longer cycle lifetime than batteries/fuel cells, have recently been receiving considerable attention.1–4 In principle, supercapacitors can be divided into two types according to the mechanism of electrical energy storage, namely, electrical double-layer capacitors (EDLCs) and pseudocapacitors.2,5 The energy storage of EDLCs is generally based on pure electrostatic charge accumulation taking place at the electrode/electrolyte interface and therefore their capacitances are strongly dependent on the surface area of this interface, while the energy storage of pseudocapacitors is accomplished by means of the reversible redox or faradaic charge transfer reactions between the electrolyte and the electroactive species on the electrode surface.Up to now, among various available electrode materials for supercapacitors, porous carbon materials, especially microporous activated carbons, are the most often used electrode materials due to their unique properties including high surface area, excellent electrical conductivity, stable physicochemical characteristics, non-toxicity, relatively low cost and easy accessibility.5–7 Since supercapacitors using carbon materials as electrodes store energy in the form of electrical double-layer capacitance, the larger specific surface area can help to get the higher charge storage capacity on the carbon/electrolyte interface.8 However, in practice, a higher specific surface area doesn't ensure a higher specific capacitance because the ions of electrolytes cannot access all of the pores of activated carbons, i.e., poor accessibility of electrolyte ions to the intrinsically small micropores arising from the closed-pores or the narrow bottle-necks.9 Moreover, ion transport within such small micropores would be kinetically limited so that the high rate capability being considered as one of the main advantages of EDLCs cannot be achieved.10 To overcome the above pore structure disadvantages of the activated carbons, special attention has been paid to the fabrication of micro–mesoporous carbons with well-balanced micro- and mesoporosity,11–15 which enable high effective specific surface area for electrical double-layer capacitance and fast ion diffusion pathways with a minimized resistance during charge/discharge process. That is to say, supercapacitors based on such porous carbons can ensure high specific capacitance and high rate capability simultaneously. For example, Zhou et al. synthesized a hierarchical mesoporous/microporous carbon with a moderate micro/meso ratio via chlorination of Ti atoms in TiC/C composites derived from a soft-templating self-assembly method. The hierarchical mesoporous/microporous carbon exhibited a high capacitance of 132 F g−1 at 0.5 A g−1 in organic electrolyte and excellent rate capability.12
Apart from high surface area and appropriate pore structures, favourable morphologies have also become central issue in the design and synthesis of porous carbons as high-performance electrode materials for supercapacitors.5 Prominent examples for this include hollow carbon nanospheres,16–19 films,20,21 nanosheets,22 and nanofibers.23 Several recent studies indicate that porous carbon microspheres represent another kind of advantageous architecture to be used for supercapacitors.24–27 In such structures, the interconnected macroporous voids resulted from the homogeneous package of carbon microspheres can serve as “ion buffering reservoirs”, which are beneficial for the improvement of electrode kinetics by decreasing the ion-transport resistance and diffusion distance from electrolyte to the surface of interior pores, and thus improve the capacity of the electrode. Besides, mixing and coating during the preparation of the electrode are relatively easier with spherical materials than with irregular-shaped particles.28 Significantly, three dimensional mesoporous/microporous carbon microsphere arrays were synthesized by a hard-templating method combined with a soft-templating self-assembly approach, and they showed a high reversible capacity of 134 F g−1 at 0.5 A g−1 with 84.4% rate performance from 0.5 to 10 A g−1 in nonaqueous electrolyte.29 It was revealed that the large capacitance and high rate capability were owing to the synergistic effect of the hierarchical micro–mesoporous structure and spherical morphology. Therefore, micro–mesoporous carbon microspheres can be considered as a good choice for supercapacitor electrode.
Another important factor influencing the capacitive performance is the surface functionalities of porous carbon materials, such as nitrogen- or oxygen-containing groups. It has been well demonstrated that such functional groups can not only enhance surface wettability of carbon materials towards electrolyte solution to improve the efficient utilization of specific surface area, but also introduce additional pseudocapacitance via reversible redox reactions as well, consequently improving the whole capacitive performance of the supercapacitor.5,30 As an example for nitrogen and oxygen rich porous carbon, high specific capacitance in the order of 440 F g−1 was recently reported.31
Generally, carbon microspheres can be obtained by direct carbon conversion of polymer microspheres. The fabrication of polymer microspheres is widely based on classical nanocasting approach,29,32 spray drying,33 solvothermal method,34,35 and precipitation polymerization,36 wherein a fussy hard templating process or a multiple-step synthesis, or the massive use of the expensive organic solvents are inevitable. Consequently, these deficiencies strongly restrict large-scale production potentials of carbon microspheres. Thus a reliable strategy to fabricate polymer microspheres is highly required. In recent years, the hydrothermal pathway has attracted more and more attention because of its merits of simplicity, low-cost production, and scale-up synthesis.37 To achieve the mass production of porous carbon microspheres required for the supercapacitor manufactures, the hydrothermal synthesis of polymer microspheres is very attractive.
Considering all aspects mentioned above, nitrogen- and oxygen-containing micro–mesoporous carbon microspheres based on the hydrothermal pathway are potentially promising materials and are expected to be particularly suitable for high-rate performance supercapacitors. Most recently, Wang et al. reported the synthesis of nitrogen and oxygen-enriched hierarchical micro–mesoporous carbon spheres (A-NHCS) with the diameter of up to 0.5–1 μm by hydrothermal reaction/carbonization/KOH activation route as the electrode materials for supercapacitors.38 The resultant A-NHCS materials exhibited a high capacitance of 356 F g−1 at 0.2 A g−1 in KOH electrolyte. However, the rate performance was still unsatisfactory (55% maintenance from 0.2 to 10 A g−1) and needed to be enhanced. Thus, the task of developing the nitrogen- and oxygen-containing porous carbon microspheres with both high capacitance and desirable rate capability using the reliable hydrothermal synthesis method still remains.
In this work, we prepared nitrogen- and oxygen-containing micro–mesoporous carbon microspheres (NO-MMCMs) through an easily scalable method combined of a self-assembly hydrothermal process and subsequent KOH activation treatment using m-aminophenol as a carbon and nitrogen co-precursor and block copolymer F127 as a soft-template. As-obtained NO-MMCMs materials integrated several advantages, such as regular microspherical morphology, high surface area, large pore volume with a well-balanced micro and mesoporosity, and moderate heteroatom content. With these merits, the NO-MMCMs exhibited excellent capacitive performance, that was, a high reversible specific capacitance, particularly high rate capability and good cycling stability in 6 M KOH electrolyte. The influences of the carbonization temperature on the crystalline structure, the porosity development, the final heteroatom content and the capacitive performance of the obtained NO-MMCMs were discussed in detail.
Experimental
Chemicals
Poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) triblock copolymer Pluronic F127 (EO106PO70EO106, Mav = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1) was purchased from Sigma-Aldrich Corp. m-Aminophenol (99 wt%) was purchased from Energy Chemical. 1,3,5-Trimethylbenzene, hexamethylenetetramine and potassium hydroxide (electronic grade, 99.9999 wt%) were purchased from Alladdin Industrial Corp. All the chemicals were used as received without further purification.
600 g mol−1) was purchased from Sigma-Aldrich Corp. m-Aminophenol (99 wt%) was purchased from Energy Chemical. 1,3,5-Trimethylbenzene, hexamethylenetetramine and potassium hydroxide (electronic grade, 99.9999 wt%) were purchased from Alladdin Industrial Corp. All the chemicals were used as received without further purification.
Synthesis of the nitrogen- and oxygen-containing micro–mesoporous carbon microspheres (NO-MMCMs)
The strategy for the fabrication of NO-MMCMs could be briefly described by the following process as shown in Scheme 1. The preparation strategy mainly comprised of the self-assembly hydrothermal synthesis of mesoporous carbon microspheres taking advantage of polymerization between m-aminophenol and formaldehyde which was produced by the hydrolysis of hexamethylenetetramine at elevated temperature, and further activation by KOH. In a typical experimental procedure, 0.275 g m-aminophenol, 0.175 g hexamethylenetetramine (HMT), 0.50 g Pluronic F127 and 0.10 g 1,3,5-trimethylbenzene (TMB) were added into 9 g deionized water. After being magnetically stirred for 2 h at room temperature, the obtained homogeneous solution was transferred into a 50 mL Teflon-lined stainless steel autoclave, and hydrothermally treated at 100 °C for 24 h. After the reaction, the autoclave was cooled down to room temperature spontaneously. The brick-red precipitate, APF (m-aminophenol formaldehyde)-F127 polymer microspheres, were collected by filtration, and then washed with ethanol as well as deionized water for several times, and finally dried at 80 °C in an oven. Subsequently, the polymer microspheres were carbonized by stepwise heating at 600, 700 or 850 °C for 3 h in a tubular furnace under a high purity argon atmosphere with a ramping rate of 1 °C min−1, to obtain mesoporous carbon microspheres.In order to prepare micro–mesoporous carbon materials, the soft-templated mesoporous carbon microspheres were chemically activated with KOH, as reported in detail elsewhere.39 In these experiments, 0.4 g of the mesoporous carbon microspheres were immersed in KOH solution (1.6 g KOH in 4 g of H2O), followed by a water evaporation step at 100 °C under vacuum. Afterwards, the impregnated samples were heated to the desired temperature of 700 °C with a heating rate of 10 °C min−1 and maintained for 1 h. After cooling in a flowing high purity argon atmosphere, the obtained products were thoroughly washed with 0.2 M HCl solution and deionized water (to assure a complete removal of residual alkali and any inorganic salts) until the filtrate became neutral, and finally dried at 80 °C for 12 h. The resultant products were designated as NO-MMCMs-X, where “NO-MMCMs” referred to nitrogen- and oxygen-containing micro–mesoporous carbon microspheres, and “X” (=600, 700, or 850 °C) denoted the carbonization temperature prior to activation.
Materials characterizations
Scanning electron microscopy (SEM) images were acquired using a field-emission Sirion scanning electron microscope. Transmission electron microscopy (TEM) experiments were conducted on a JEOL 2100 microscope at operating voltage of 200 kV. The samples were dispersed in ethanol and dropped on laces support films. Wide angle X-ray diffraction measurements were taken on an X'Pert PRO MPD X-ray diffractometer using Ni-filtered Cu-Kα radiation source. The X-ray photoelectron spectroscopy (XPS) characterizations were carried out on an ESCALAB250Xi spectrometer with Al Kα radiation (hv = 1486.6 eV). Nitrogen adsorption/desorption isotherms were measured at 77 K on a 3H-2000PM1 analyzer (Beishide, China). Before the measurements of the sorption isotherms, the samples were degassed at 300 °C for 4 h under vacuum. The specific surface area (SBET) was calculated using the Brunauer–Emmett–Teller (BET) method based on the adsorption data of corresponding isotherm in a relative pressure range from 0.04 to 0.32. The total pore volume (Vt) was calculated from the adsorbed amount of nitrogen at a relative pressure of P/P0 = 0.995, and the micropore volume (Vmi) was determined by using the t-plot method. The mesopore size distributions were calculated from the adsorption branch of the isotherm using the Barrett–Joyner–Halenda (BJH) model, whereas the micropore size distributions were obtained by using the Harvath–Kawazoe (HK) method.Electrochemical measurements
The working electrodes were fabricated by coating a homogeneous slurry containing the activated materials (as-prepared NO-MMCMs), acetylene black and polytetrafluoroethylene (PTFE) binder with the weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in a required amount of ethanol onto a 1 cm × 1 cm nickel foam and a circular nickel foam with diameter of 1.5 cm, followed by drying overnight at 100 °C in a vacuum oven. Prior to the electrochemical measurements, the electrodes were pressed under a pressure of 10 MPa. The mass loading of the active materials in each electrode was about 5 mg. The electrochemical measurements were performed in both the three-electrode configuration and the two-electrode configuration with the 6 M KOH aqueous solution serving as the electrolyte. In a conventional three-electrode system, the NO-MMCMs based electrode, a platinum foil and a Hg/HgO electrode were utilized as the working, counter and reference electrodes, respectively. In a two-electrode system, the symmetric supercapacitor device was assembled with a 2025-type coin cell. Before fabrication of the cell, the two NO-MMCMs based electrodes with the same size and sample weight were first prewetted with 6 M KOH electrolyte, and separated by a thin polypropylene film, and then packed into a two-electrode cell. Cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) were measured on a CHI 660D electrochemical workstation (Chenhua Instruments Inc., Shanghai). Cyclic voltammetry (CV) tests were performed within a potential window range of −1.0 to 0 V at various scan rates ranging from 5 to 200 mV s−1. Electrochemical impedance spectroscopy (EIS) measurements were recorded from 100 kHz to 10 mHz at the open-circuit potential with an alternate current amplitude of 5 mV. Galvanostatic charge–discharge (GCD) experiments were carried out in the potential window between −1.0 and 0 V at different current densities of 0.1–20 A g−1. Long-term cycling stability experiments were performed on a supercapacitor test system (Neware, Guangdong, China).
10 in a required amount of ethanol onto a 1 cm × 1 cm nickel foam and a circular nickel foam with diameter of 1.5 cm, followed by drying overnight at 100 °C in a vacuum oven. Prior to the electrochemical measurements, the electrodes were pressed under a pressure of 10 MPa. The mass loading of the active materials in each electrode was about 5 mg. The electrochemical measurements were performed in both the three-electrode configuration and the two-electrode configuration with the 6 M KOH aqueous solution serving as the electrolyte. In a conventional three-electrode system, the NO-MMCMs based electrode, a platinum foil and a Hg/HgO electrode were utilized as the working, counter and reference electrodes, respectively. In a two-electrode system, the symmetric supercapacitor device was assembled with a 2025-type coin cell. Before fabrication of the cell, the two NO-MMCMs based electrodes with the same size and sample weight were first prewetted with 6 M KOH electrolyte, and separated by a thin polypropylene film, and then packed into a two-electrode cell. Cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) were measured on a CHI 660D electrochemical workstation (Chenhua Instruments Inc., Shanghai). Cyclic voltammetry (CV) tests were performed within a potential window range of −1.0 to 0 V at various scan rates ranging from 5 to 200 mV s−1. Electrochemical impedance spectroscopy (EIS) measurements were recorded from 100 kHz to 10 mHz at the open-circuit potential with an alternate current amplitude of 5 mV. Galvanostatic charge–discharge (GCD) experiments were carried out in the potential window between −1.0 and 0 V at different current densities of 0.1–20 A g−1. Long-term cycling stability experiments were performed on a supercapacitor test system (Neware, Guangdong, China).
For the three-electrode system, the specific capacitance of the NO-MMCMs can be calculated according to GCD tests by the following equation:
| C = IΔt/(ΔVm) | (1) |
Based on the EIS data, the specific capacitance of the NO-MMCMs can be calculated through the following formula:
| C = −1/(2πfZ′′m) | (2) |
For the two-electrode system, the specific capacitance, energy density and power density of the assembled supercapacitor can be calculated based on GCD tests by the following equations:
| Cm = IΔt/(ΔVm) | (3) |
| Csp = 4Cm | (4) |
| E = 0.5CmΔV2/3.6 | (5) |
| P = 3600E/Δt | (6) |
The coulombic efficiency can be calculated on the basis of the following equation:
| η (%) = td/tc × 100% | (7) |
Results and discussion
Structural and chemical properties of the NO-MMCMs
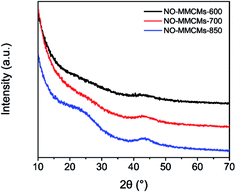
The surface morphology of the NO-MMCMs was investigated by means of SEM technique. Fig. 1 shows SEM image of typical sample NO-MMCMs-600. SEM image of NO-MMCMs-600 clearly demonstrates that the activated carbon particles have regular spherical morphology with diameter of 0.5–8 μm and the morphological yield of the microspheres is near 100%. Besides, plenty of three-dimensionally interconnected macropores, which are constructed by interparticle voids, can be observed. The structural study was further carried out by the TEM measurement. Fig. 2a–c show the TEM images of NO-MMCMs-600 with different size. The regular spherical shape of NO-MMCMs-600 is in agreement with the SEM observation. The porosity of NO-MMCMs-600 is composed of randomly distributed, interconnected, and worm-like pores, as illustrated by the HRTEM image in Fig. 2d. The interconnectivity of pores would facilitate the electrolyte ion diffusion in the material. The crystalline structure of the NO-MMCMs was identified by XRD analysis. All the XRD patterns of the NO-MMCMs in Fig. 3 show two broad and weak diffraction peaks at 2θ values of approximately 23.8° and 43.6° that belong to the (002) and (100) lattice planes of amorphous carbons, respectively, revealing the amorphous structure of the carbon microspheres.25 Notably, the intensities of these two peaks gradually weaken from NO-MMCMs-850 to NO-MMCMs-600, indicating that deeper activation extent has promoted the increase in structural defects and disordered section in NO-MMCMs-700 and NO-MMCMs-600 samples (Fig. 3).Nitrogen adsorption/desorption isotherm measurements were conducted to analyze the textural characteristics of the NO-MMCMs materials. Fig. 4a shows the nitrogen adsorption/desorption isotherms of various NO-MMCMs samples. The large nitrogen uptakes below the relative pressure of P/P0 = 0.1 and the continuous increase of nitrogen adsorption in the relative pressure range from 0.1 to 0.9 are observed for all the carbon microspheres, evidently suggesting the presence of micro/mesoporous structures.14 This can be further verified by the pore size distribution curves displayed in Fig. 4b and c, which reveal that these carbons have a hierarchical pore structure made up of narrow micropores with the peak of pore width centered at about 0.62 nm and small mesopores with pore size in the range of 2–5 nm. In addition, the isotherms exhibit a slight upward tendency at high relative pressure from 0.9 to 1.0, which is related to the interparticle voids between the carbon microspheres.40 The pore parameters of the NO-MMCMs calculated from N2 adsorption/desorption isotherms are summarized in Table 1. As can be seen, the NO-MMCMs-600 possesses BET surface area as high as 3203 m2 g−1, which is larger than 2818 m2 g−1 of NO-MMCMs-700 and 2012 m2 g−1 of NO-MMCMs-850, and is comparable to 3432 m2 g−1 of previously reported polypyrrole-derived activated carbons,41 being one of the highest values ever reported for carbon materials. The total pore volume and also the mesopore volume show the decreased tendency with an increase in carbonization temperature. These results reveal that carbonization temperature prior to activation plays a crucial role in controlling the porosity development during the KOH activation process. The KOH activation is more efficient for mesoporous carbon derived from the polymer microspheres carbonized at 600 °C as compared to those carbonized at 700 and 850 °C, probably because the carbonaceous framework of the former is less rigid as a result of low temperature carbonization and consequently more vulnerable to the activation process, which leads to the higher specific surface area of NO-MMCMs-600.32,39,42 According to the previously reported literatures,43,44 in the case of the lower carbonization temperatures (below 600 °C), the KOH activation of this kind of phenolic resin-based mesoporous carbons gives purely micropores material, which is not the main goal of this project. We therefore focus our study on the carbon samples subjected to carbonization at or above 600 °C.
| Sample | Pore parameters | Element content from XPS | ||||||
|---|---|---|---|---|---|---|---|---|
| SBETa [m2 g−1] | Vtb [cm3 g−1] | Vmic [cm3 g−1] | Vmed [cm3 g−1] | C (at%) | N (at%) | O (at%) | N + O (at%) | |
| a BET surface area.b Total pore volume.c Micropore volume determined by using the t-plot method.d Mesopore volume obtained by subtracting Vmi from Vt. | ||||||||
| NO-MMCMs-600 | 3203 | 1.93 | 1.43 | 0.50 | 93.36 | 1.29 | 5.35 | 6.64 |
| NO-MMCMs-700 | 2818 | 1.48 | 1.04 | 0.44 | 93.60 | 0.97 | 5.43 | 6.40 |
| NO-MMCMs-850 | 2012 | 0.99 | 0.69 | 0.30 | 94.24 | 1.87 | 3.88 | 5.76 |
In attempts to verify whether nitrogen and oxygen remain in the carbon matrix, XPS studies were performed. The survey spectra of NO-MMCMs in Fig. 5a show that there exist three peaks around 285, 400, and 532 eV that correspond to C1s, N1s and O1s peaks, respectively,45 demonstrating the successful incorporation of nitrogen and oxygen into the carbon frameworks. From the XPS survey spectra, the relative surface contents of nitrogen and oxygen are calculated to be 1.87 at% and 3.88 at% for NO-MMCMs-850, 0.97 at% and 5.43 at% for NO-MMCMs-700, 1.29 at% and 5.35 at% for NO-MMCMs-600, respectively, as shown in Table 1. The chemical environments of nitrogen and oxygen elements on the surface of the carbon microspheres are further investigated by means of XPS analysis and the resulting high resolution N1s and O1s spectra are presented in Fig. 5b and c. In the case of N1s, four different peaks centered at 398.5 ± 0.2 eV, 400.5 ± 0.2 eV, 401.2 ± 0.2 eV and 402.9 ± 0.2 eV are detected, corresponding to pyridinic-N (N-6), pyrrolic-/pyridonic-N (N-5), quaternary-N (N-Q) and pyridine-N-oxide (N-X), respectively.46 It is believed that N-6 and N-5 with nitrogen located at the edge of graphene layers are quite favorable for the pseudocapacitive faradic reactions, which is very important for nitrogen-doped carbon materials to increase the capacitance.47 The relative amount (%) of each nitrogen component in the different samples is summarized in Table S1.† N-6 and N-5 are the dominant form of nitrogen in all samples, with the total contents of the two in the range of 55–77%, indicating that nitrogen functional groups can effectively provide pseudocapacitance. NO-MMCMs-600 presents the highest N-6 and N-5 content of around 77%. In the case of O1s, deconvoluted O1s spectra contain four characteristic peaks at 531.3 ± 0.2 eV, 532.4 ± 0.3 eV, 533.9 ± 0.5 eV and 536.3 ± 0.2 eV, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (O-I), C–OH/C–O–C (O-II), O–C
O (O-I), C–OH/C–O–C (O-II), O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (O-III) and chemisorbed oxygen and/or water (O-IV), respectively.48 Among these oxygen functional groups, the carbonyl group (C
O (O-III) and chemisorbed oxygen and/or water (O-IV), respectively.48 Among these oxygen functional groups, the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) is considered to be electrochemically active in an alkaline electrolyte to provide main pseudocapacitance, during which the carbonyl group (C
O) is considered to be electrochemically active in an alkaline electrolyte to provide main pseudocapacitance, during which the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stores and releases an electron without ion exchange.49 The critically important role of the carbonyl groups within functionalized-graphene- or activated-carbon-nanospheres-based electrodes in developing high-performance supercapacitors has been reported.49,50 The quantitative analyses (see Table S1†) of oxygen species for all samples reveal that the carbonyl groups are around 26–48% of the total oxygen atoms. In addition to the pseudocapacitance contribution, hydrophilic N, O-species also play an important role in improving the wettability of carbon materials, which facilitates the accessibility of the electrolyte ions.
O) stores and releases an electron without ion exchange.49 The critically important role of the carbonyl groups within functionalized-graphene- or activated-carbon-nanospheres-based electrodes in developing high-performance supercapacitors has been reported.49,50 The quantitative analyses (see Table S1†) of oxygen species for all samples reveal that the carbonyl groups are around 26–48% of the total oxygen atoms. In addition to the pseudocapacitance contribution, hydrophilic N, O-species also play an important role in improving the wettability of carbon materials, which facilitates the accessibility of the electrolyte ions.
 | ||
| Fig. 5 (a) XPS survey spectra, and high resolution (b) O1s and (c) N1s XPS spectra of NO-MMCMs-600, NO-MMCMs-700 and NO-MMCMs-850. | ||
Electrochemical performance
The electrochemical performances of as-prepared NO-MMCMs as supercapacitor electrodes materials have been systematically investigated by cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) techniques in a conventional three-electrode system using 6 M KOH solution as the electrolyte. Fig. 6a shows the CV curves of the NO-MMCMs at a scan rate of 5 mV s−1 with a potential range of from −1.0 to 0 V (vs. Hg/HgO). As can be seen, the CV curves of all the samples display quasi-rectangular shapes superimposed with well-broadened humps located at low potential range, indicating that their capacitive responses come from the electric double layer formation and redox reactions related to the nitrogen and/or oxygen functional groups on the surface of the electrode.51 Comparatively, the humps in the CV curves become less readily identified with the increasing of carbonization temperature from 600 to 850 °C due to the decreased amounts of active nitrogen and oxygen heteroatoms.48,52 Generally, the surrounded area by the CV profile is directly proportional to the specific capacitance for supercapacitors at the same scan rate.53 Noteworthy, the area of the CV curve for NO-MMCMs-600 is the largest among all the tested carbon materials, suggesting the highest specific capacitance. Fig. 6b presents the rate-dependent CV curves for the NO-MMCMs-600 at different scan rates ranging from 5 to 100 mV s−1. It can be clearly seen that the CV curve maintains rectangular-like shape and undergoes no obvious distortion even at a high scan rate of 100 mV s−1, which shows that the NO-MMCMs-600 possesses rapid ions transfer-diffusion and good rate capability.22 Fig. S1† also reveals rate-dependent CV curves of the NO-MMCMs-700 and the NO-MMCMs-850, which are the same as that of the NO-MMCMs-600.Fig. 6c presents the GCD curves of the NO-MMCMs at a low discharge rate of 0.1 A g−1. It is clear that all of the GCD curves exhibit nearly symmetric triangle shapes, suggesting that such electrodes possess excellent electrochemical reversibility. Unlike linear characteristics, the small deviation to linearity can be easily noticed for all the discharge branches. It further confirms the presence of the pseudocapacitance effect attributed to the quick redox reaction during the charging/discharging processes, which agrees well with the CV measurement analysis.54 The GCD measurements at enhanced current density are utilized to further examine the rate capability of the NO-MMCMs. Fig. 6d displays a plot of the specific capacitance as a function of the current density. As calculated, the specific capacitance is 309 F g−1 at the current density of 0.1 A g−1 for NO-MMCMs-600, which is significantly higher than those of the other NO-MMCMs samples under the same conditions (the specific capacitance values for NO-MMCMs-700 and NO-MMCMs-850 are 266 F g−1 and 197 F g−1, respectively). Among the carbon spheres, NO-MMCMs-600 presents the best performance presumably because it combines the highest surface area (3203 m2 g−1) with the highest N, O-doping level (6.64 at%) which makes the redox reaction full well at low current density. On increasing the current density, the specific capacitance decreases for these carbon spheres. Moreover, the variation tendency of specific capacitance in the range of 0.1–20 A g−1 is divided into three parts. One, the specific capacitance declines sharply at low current densities below 1 A g−1, which can be ascribed to the presence of micropores and the attenuation of pseudocapacitance.55–57 On the other hand, a slow decrease in the specific capacitance can be observed at medium current densities range from 1 to 5 A g−1, followed by a nearly plateau at high current densities range from 5 to 20 A g−1, which are related to the mesopores and macropores between the carbon microspheres.55,56 It is noteworthy to mention that the specific capacitance of NO-MMCMs-600 can still retain 229 F g−1 with a retention ratio of 74% when the current density increases from 0.1 to 20 A g−1, indicating good property at high current density and excellent rate capability, which is highly comparable with NO-MMCMs-700 (189 F g−1 and 71%) and NO-MMCMs-850 (142 F g−1 and 72%). Table 2 compares the capacitive performance of the NO-MMCMs-600 electrode with those of electrodes based on carbon spheres reported in the recent literatures.,6,24–27,34–36,38,50,51,58–60 and lists the major characteristics of each report. At high current density of 20 A g−1, NO-MMCMs-600 shows a much superior high-rate capacitive performance to most reported carbon spheres. From the comparison of the capacitive performance of other reported carbon spheres in the literatures, it clearly reveals that the lower microporosity, the lower specific capacitance is achieved at high current densities.6,26,27,58,59 However, extensive microporosity could not lead to high rate capability.38 The nitrogen and sulfur codoped porous carbon microspheres35 exhibit better rate capability than nitrogen-containing carbon microspheres,25 porous nitrogen-doped carbon microspheres,34 nitrogen-doped hierarchical porous carbon microspheres,36 and activated carbon nanospheres50 despite lower specific surface area and pore volume, and it could be explained by the high contents of nitrogen and oxygen and small amounts of sulfur which not only enhance the hydrophilicity of the materials that can reduce the diffusion resistance of the electrolyte ions, but also introduce the pseudocapacitance.35 It shows that the N, O-doping content of NO-MMCMs-600 in this work is lower while the surface area is much higher than that of nitrogen-containing carbon microspheres,25 porous nitrogen-doped carbon microspheres,34 nitrogen and sulfur codoped porous carbon microspheres,35 and nitrogen-doped hierarchical porous carbon microspheres.36 Therefore, it is believed that the high specific surface area and well-balanced micro/mesoporosity of the NO-MMCMs-600 play the most important role in improving the rate performance.
| Carbon material | SBET [m2 g−1] | Vtotal [m3 g−1] | Vmeso [m3 g−1] | N (at%) | O (at%) | Capacitance (F g−1) | Rate performance | Electrolyte | Reference |
|---|---|---|---|---|---|---|---|---|---|
| a Nitrogen-containing ultramicroporous carbon nanospheres.b Ultrathin hollow mesoporous carbon nanospheres.c Small mesopores-enriched porous carbon microspheres.d Nitrogen-containing carbon microspheres.e Mesoporous carbon microspheres.f Hierarchical porous carbon microspheres.g Porous nitrogen-doped carbon microspheres.h Nitrogen and sulfur codoped porous carbon microspheres.i Nitrogen-doped hierarchical porous carbon microspheres.j Nitrogen-doped hierarchical carbon spheres.k Activated carbon nanospheres.l Nitrogen-doped hollow graphitic carbon spheres.m Mesoporous size controllable carbon microspheres.n Micro- and mesoporous carbon spheres.o Nitrogen-rich microporous carbon spheres. | |||||||||
| N-UCNs4.50a | 1439 | 2.12 | 1.60 | 1.21 | 4.29 | 376 (0.5 A g−1) | 130 F g−1 at 20 A g−1 | 6 M KOH | 6 |
| HMCNsb | 568 | 1.63 | — | — | — | 253 (1.0 A g−1) | 111 F g−1 at 60 A g−1 | 6 M KOH | 18 |
| PCMsc | 1163 | 0.827 | — | — | — | 172 (1.0 A g−1) | 155 F g−1 at 16 A g−1 | 6 M KOH | 24 |
| NCMs-700d | 403 | 0.35 | — | 5.94 | 3.88 | 228 (1.0 A g−1) | 189 F g−1 at 5 A g−1 | 6 M KOH | 25 |
| MCMs-3e | 1010 | 0.853 | 0.57 | — | — | 171 (1.0 A g−1) | 157 F g−1 at 10 A g−1 | 6 M KOH | 26 |
| HPCMSs-2f | 709 | 0.88 | 0.61 | — | — | 221 (1.0 A g−1) | 126 F g−1 at 5 A g−1 | 6 M KOH | 27 |
| A-PNCMg | 1303 | 0.44 | — | 3.2 | 9.7 | 282 (0.5 A g−1) | 154 F g−1 at 20 A g−1 | 6 M KOH | 34 |
| NS-PCMSs-TH-750h | 413.8 | 0.197 | — | 3.92 | 20 | 295 (0.1 A g−1) | 245 F g−1 at 10 A g−1 | 6 M KOH | 35 |
| PM-600-1.0i | 568.2 | 0.33 | — | 5.0 | 9.0 | 278 (0.1 A g−1) | 147 F g−1 at 10 A g−1 | 1 M H2SO4 | 36 |
| A–NHCS–2j | 1101 | 0.49 | 0.11 | 9.3 | — | 356 (0.2 A g−1) | 196 F g−1 at 10 A g−1 | 6 M KOH | 38 |
| CK23k | 1533 | 1.162 | 0.681 | — | 9.7 | 262 (0.2 A g−1) | 204 F g−1 at 20 A g−1 | 6 M KOH | 50 |
| NHGCSsl | 753 | — | — | — | — | 306 (0.1 A g−1) | 215 F g−1 at 4 A g−1 | 2 M H2SO4 | 51 |
| MCM-70-5.5m | 687 | 0.59 | 0.38 | — | — | 268 (1.0 A g−1) | 163 F g−1 at 20 A g−1 | 6 M KOH | 58 |
| MMCSs-3n | 1620 | 1.04 | 0.63 | — | — | 314 (0.5 A g−1) | 151 F g−1 at 15 A g−1 | 6 M KOH | 59 |
| NMCSso | 1929 | 0.85 | — | 11.8 | 7.2 | 365 (0.5 A g−1) | 230 F g−1 at 10 A g−1 | 6 M KOH | 60 |
| NO-MMCMs-600 | 3203 | 1.93 | 0.50 | 1.29 | 5.35 | 309 (0.1 A g−1) | 229 F g−1 at 20 A g−1 | 6 M KOH | This work |
In order to investigate the facilitated ion- and electron-transport behavior within the NO-MMCMs electrodes, EIS was recorded over a frequency range from 100 kHz to 0.01 Hz at open circuit potential, and corresponding test results were shown in Fig. 6e and f. According to Fig. 6e with an enlarged view provided in the inset, the Nyquist plots for all the samples exhibit four distinct parts, which consist of an intercept on the real impedance axis (Z′) at sufficiently high frequency, followed by a depressed semicircle in the high-to-medium frequency region, a 45° inclined line in the medium frequency range and an almost vertical line in the low frequency region. To be more detailed, at very high frequency, the intercept of Nyquist plot on the real impedance axis represents the equivalent series resistance (ESR). It is a combination of the electrolyte resistance, the intrinsic resistance of the active material, and the contact resistance at the interface of the active material/current collector, and can be associated with the surface properties of the porous electrode.61,62 Although NO-MMCMs-600 has lower degree of graphitization and more surface oxygen content compared to the two other counterparts, the ESR of the NO-MMCMs-600 (1.09 Ω) is lower than that of NO-MMCMs-700 (1.15 Ω) and NO-MMCMs-850 (1.20 Ω), mainly owing to the better surface properties of the NO-MMCMs-600 that will lead to a lower contact resistance. The delicate difference of ESR suggests that ESR may not be the most major determinant for the difference of the overall internal resistance of the NO-MMCMs electrodes. The semicircle in the high-to-medium frequency region corresponds to the charge transfer resistance (Rct) and the semicircle diameter reflects the magnitude of Rct. The Nyquist plot of NO-MMCMs-850 exhibits the largest diameter of semicircle, and the diameter of semicircle obviously decreases with the order of NO-MMCMs-850 > NO-MMCMs-700 > NO-MMCMs-600. The decreasing trend of Rct from NO-MMCMs-850 to NO-MMCMs-600 is associated with the increased contact area at the interface between the electrode and the electrolyte attributing to the improved wettability of the electrodes and developed micro/mesopore structure, which is in favor of charge transfer process.23,63 This result demonstrates that higher accessibility of electrolyte to NO-MMCMs will achieve lower Rct, which is one of the key determinants for the overall internal resistance of the NO-MMCMs electrodes. The inclined line with a slope of about 45° in the middle frequency region is ascribed to the Warburg impedance (Zw) and is representative of ion diffusion into the electrode materials.64 NO-MMCMs-600 has a relatively shorter projected length of the Warburg-type line on the real impedance axis compared to the other two samples, suggesting the faster ion diffusion capability. This phenomenon can be explained by the high porosity and high surface area of NO-MMCMs-600 that enable smooth diffusion. The vertical line at low frequency region is a result of the frequency dependence of ion diffusion at the carbon/electrolyte interface, and reflects the ion diffusive resistivity at electrode/electrolyte interface. A more vertical linear shape in the low frequency region observed for NO-MMCMs-600 indicates that it behaves more like an ideal capacitive behavior with better pore accessibility for electrolyte ions and the lower ion diffusive resistivity at carbon material/electrolyte interface.31,65 This is due to higher amount of the nitrogen- and oxygen-containing functionalities which may enhance the wettability of electrolyte towards electrode surface of the NO-MMCMs-600 that decreases the contact resistance of the interface between carbon material and electrolyte.66 In a word, NO-MMCMs-600 demonstrates an efficient pathway for charge transfer and electrolyte ion movement throughout the carbonaceous matrix. Fig. 6f shows the variation in the ratio of retained capacitance versus frequency. As displayed, the capacitances of all samples decrease gradually at the frequencies below 1 Hz, and decrease sharply in the 1–100 Hz range and trend towards to zero in the higher frequency region. At low frequencies, the ions have adequate time to diffuse into the pores of carbon microspheres and access to more electrode surfaces, leading to high capacitance retentions. However, at higher frequencies, ions penetration inside the inner pores becomes difficult and consequently only partial pore surfaces of carbon microspheres are accessible for electrolyte ions, resulting in sharp decrease in the capacitances. The NO-MMCMs-600 presents the slowest capacitance drop, and at a characteristic frequency of 1 Hz (charged within 1 s), it still can remain 76% of its maximum capacitance, further confirming fast ion and electrical charge transfer at high charge/discharge rates within the NO-MMCMs-600 electrode, which is important for achieving high-rate performance. Since cycle life is an important evaluation index for the practical application of supercapacitors especially if pseudocapacitance exists, the cycle stability of the NO-MMCMs-600 was studied through a long-term charge/discharge cycling test at a current density of 10 A g−1 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The results in Fig. 6g reveal that 95% of the original specific capacitance is still maintained after 10
000 cycles. The results in Fig. 6g reveal that 95% of the original specific capacitance is still maintained after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles with a coulombic efficiency of almost 100%, which evidently show that the NO-MMCMs-600 has excellent electrochemical stability and high reversibility that will be highly desirable in practical applications.
000 cycles with a coulombic efficiency of almost 100%, which evidently show that the NO-MMCMs-600 has excellent electrochemical stability and high reversibility that will be highly desirable in practical applications.
Further, a two-electrode symmetric coin cell was also assembled to evaluate the electrochemical performance of the NO-MMCMs-600 in 6 M KOH electrolyte. Fig. 7a demonstrates the CV curves of the NO-MMCMs-600//NO-MMCMs-600 symmetric supercapacitor at various scan rates from 5 to 200 mV s−1. The CV curve can still retain the nearly rectangular shape even at a high scan rate of 200 mV s−1, implying a typical characteristic of double-layer capacitor performance and the superior ion response. Fig. 7b shows the GCD profiles of the symmetric supercapacitor at different current densities from 0.1 to 20 A g−1. The GCD curves are almost symmetric triangular shapes, revealing good capacitive characteristic and electrochemical reversibility. Fig. 7c shows the specific capacitance for a single NO-MMCMs-600 electrode calculated from the GCD at various current densities. When the current density increases to from 0.1 to 20 A g−1, the capacitance values slightly decrease from 261 to 200 F g−1, retaining about 77% of the initial capacitance, which is indicative of an outstanding rate performance of NO-MMCMs-600. The performance is apparently superior to that of ultrahigh-surface-area hollow carbon nanospheres (144 F g−1 at 15 A g−1),17 and hollow graphene spheres (163 F g−1 at 5 A g−1),19 and highly porous carbon spheres from hemp stem hemicellulose (240 F g−1 at 0.1 A g−1).57 Fig. 7d depicts the Ragone plot related to energy and power densities of the symmetric supercapacitor. It is found that the energy density can reach 9.1 W h kg−1 at a current density of 0.1 A g−1, and still remains 4.5 W h kg−1 with a power density of 8010 W kg−1 at a current density of 20 A g−1. The energy density is higher than that of the commercial carbon supercapacitors (the energy density value is generally 3–5 W h kg−1).26 Fig. 7e shows the long-term cycling stability of the symmetric supercapacitor examined at a current density of 5 A g−1. After 9000 charge/discharge cycles, the symmetric cell can still retain 98% of its initial capacitance and a coulombic efficiency of 100%, evidencing the excellent cycling stability and very high reversibility of the NO-MMCMs-600 material.
Hence, from the above discussions, it can be seen that the NO-MMCMs-600 electrode material possesses an excellent high-rate electrochemical performance. This behavior is mainly attributable to the combined effect of: (i) the high-surface-area hierarchical microporous/mesoporous structure, which can not only provide a large accessible surface area for effective charge accumulation, but also low-resistant pathways for rapid transport of the electrolyte ions within the porous carbon microspheres, (ii) the space between the carbon microspheres which serves as “ion buffering reservoirs”, reducing the diffusion distances of electrolyte ions to the internal carbon surfaces, and (iii) a considerable amount of nitrogen and oxygen surface functional groups on the carbon skeleton which are quite beneficial for the improvement of electrode wettability that increases the accessibility of micropores for electrolyte ions at high rate.
Conclusions
To summarize, we have prepared nitrogen- and oxygen-containing micro–mesoporous carbon microspheres (NO-MMCMs) through a self-assembly hydrothermal method employing m-aminophenol as a carbon and nitrogen co-precursor and block copolymer F127 as a soft template, followed by carbonization and further KOH activation. The as-obtained NO-MMCMs materials combine the characteristics of large surface area, hierarchical porosity, and well-defined spherical morphology as well as reasonable amount of nitrogen and oxygen functional groups. Benefiting from these unique structural and textural properties, the NO-MMCMs demonstrate excellent electrochemical performances as electrode materials for supercapacitors. A typical sample, NO-MMCMs-600 which has high specific surface area (up to 3203 m2 g−1), large pore volume (up to 1.93 cm3 g−1) with a well-balanced micro- and mesoporosity, and moderate heteroatom content (6.64 at%) as electrode materials in 6 M KOH electrolyte, shows a high specific capacitance of 309 F g−1 at a current density of 0.1 A g−1 in a three-electrode system. Even at the high current density of 20 A g−1, it still retains relatively high specific capacitance of 229 F g−1. Moreover, the material also exhibits an excellent cycling stability with only 5% capacitance loss after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 consecutive charge/discharge cycles. For a two-electrode cell, the NO-MMCMs-600 displays a specific capacitance of 261 F g−1, an outstanding rate capability (retention of 77% from 0.1 to 20 A g−1) and good stability of 98% capacitance retention after 9000 cycles. The high current charge and discharge capability, indication of high power density, highlights the great potential for the application of NO-MMCMs-600 as electrode materials in supercapacitors which meet the need of high power density.
000 consecutive charge/discharge cycles. For a two-electrode cell, the NO-MMCMs-600 displays a specific capacitance of 261 F g−1, an outstanding rate capability (retention of 77% from 0.1 to 20 A g−1) and good stability of 98% capacitance retention after 9000 cycles. The high current charge and discharge capability, indication of high power density, highlights the great potential for the application of NO-MMCMs-600 as electrode materials in supercapacitors which meet the need of high power density.
Acknowledgements
We gratefully acknowledge the support of this research work by the Fundamental Research Funds for the Central Universities (2572014DB03), Natural Science Foundation of Heilongjiang Province of China (B201402), Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province (LBH-Q14004), and the Science and Technology Research Foundation of Heilongjiang Province Department of Education (12513005). Furthermore, we thank Prof. Ying Gao and Pengjian Zuo for characterization assistance.References
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- Y. P. Zhai, Y. Q. Dou, D. Y. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828–4850 CrossRef CAS PubMed.
- M. H. Yu, W. T. Qiu, F. X. Wang, T. Zhai, P. P. Fang, X. H. Lu and Y. X. Tong, J. Mater. Chem. A, 2015, 3, 15792–15823 CAS.
- L. B. Dong, C. J. Xu, Y. Li, Z. H. Huang, F. Y. Kang, Q. H. Yang and X. Zha, J. Mater. Chem. A, 2016, 4, 4659–4685 CAS.
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- W. J. Lu, M. X. Liu, L. Miao, D. Z. Zhu, X. Wang, H. Duan, Z. W. Wang, L. C. Li, Z. J. Xu, L. H. Gan and L. W. Chen, Electrochim. Acta, 2016, 205, 132–141 CrossRef CAS.
- C. R. Castro, C. Schütter, S. Passerini and A. Balducci, Electrochim. Acta, 2016, 206, 452–457 CrossRef.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- Y. F. Song, S. Hu, X. L. Dong, Y. G. Wang, C. X. Wang and Y. Y. Xia, Electrochim. Acta, 2014, 146, 485–494 CrossRef CAS.
- A. B. Fuertes, G. Lota, T. A. Centeno and E. Frackowiak, Electrochim. Acta, 2005, 50, 2799–2805 CrossRef CAS.
- K. S. Xia, Q. M. Gao, J. H. Jiang and J. Hu, Carbon, 2008, 46, 1718–1726 CrossRef CAS.
- D. D. Zhou, Y. J. Du, Y. F. Song, Y. G. Wang, C. X. Wang and Y. Y. Xia, J. Mater. Chem. A, 2013, 1, 1192–1200 CAS.
- E. G. Calvo, N. F. Lorenzo, J. A. Menéndez and A. Arenillas, Microporous Mesoporous Mater., 2013, 168, 206–212 CrossRef CAS.
- A. B. Fuertes and M. Sevilla, Carbon, 2015, 94, 41–52 CrossRef CAS.
- M. J. Kim, I. Oh and J. Kim, J. Power Sources, 2016, 307, 715–723 CrossRef CAS.
- J. P. Han, G. Y. Xu, B. Ding, J. Pan, H. Dou and D. R. MacFarlane, J. Mater. Chem. A, 2014, 2, 5352–5357 CAS.
- F. Xu, Z. W. Tang, S. Q. Huang, L. Y. Chen, Y. R. Liang, W. C. Mai, H. Zhong, R. W. Fu and D. C. Wu, Nat. Commun., 2015, 6, 7221–7230 CrossRef PubMed.
- Y. H. Dai, H. Jiang, Y. J. Hu, Y. Fu and C. Z. Li, Ind. Eng. Chem. Res., 2014, 53, 3125–3130 CrossRef CAS.
- J. Y. Cao, Y. M. Wang, P. Xiao, Y. C. Chen, Y. Zhou, J. H. Ouyang and D. C. Jia, Carbon, 2013, 389–391 CrossRef.
- J. L. Liu, J. Sun and L. Gao, J. Phys. Chem. C, 2010, 114, 19614–19620 CAS.
- J. L. Liu, L. L. Zhang, H. B. Wu, J. Y. Lin, Z. X. Shen and X. W. Lou, Energy Environ. Sci., 2014, 7, 3709–3719 CAS.
- H. Peng, G. F. Ma, K. J. Sun, Z. G. Zhang, Q. Yang and Z. Q. Lei, Electrochim. Acta, 2016, 190, 862–871 CrossRef CAS.
- X. D. Tian, N. Zhao, Y. Song, K. Wang, D. F. Xu, X. Lia, Q. G. Guo and L. Liu, Electrochim. Acta, 2015, 185, 40–51 CrossRef CAS.
- Z. S. Li, D. H. Li, Z. H. Liu, B. L. Li, C. Y. Ge and Y. P. Fang, Electrochim. Acta, 2015, 158, 237–245 CrossRef CAS.
- D. Z. Zhu, Y. W. Wang, L. H. Gan, M. X. Liu, K. Cheng, Y. H. Zhao, X. X. Deng and D. G. Sun, Electrochim. Acta, 2015, 158, 166–174 CrossRef CAS.
- W. Xiong, M. X. Liu, L. H. Gan, Y. K. Lv, Y. Li, L. Yang, Z. J. Xu, Z. X. Hao, H. L. Liu and L. W. Chen, J. Power Sources, 2011, 196, 10461–10464 CrossRef CAS.
- Q. L. Zhao, X. Y. Wang, C. Wu, J. Liu, H. Wang, J. Gao, Y. W. Zhang and H. B. Shu, J. Power Sources, 2014, 254, 10–17 CrossRef CAS.
- O. J. Kwon, Y. S. Jung, J. H. Kim and S. M. Oh, J. Power Sources, 2004, 125, 221–227 CrossRef CAS.
- D. D. Zhou, H. J. Liu, Y. G. Wang, C. X. Wang and Y. Y. Xia, J. Mater. Chem., 2012, 22, 1937–1943 RSC.
- L. Zhao, L. Z. Fan, M. Q. Zhou, H. Guan, S. Y. Qiao, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 5202–5206 CrossRef CAS PubMed.
- J. Li, K. Liu, X. Gao, B. Yao, K. Y. Huo, Y. L. Cheng, X. F. Cheng, D. C. Chen, B. Wang, W. M. Sun, D. Ding, M. L. Liu and L. Huang, ACS Appl. Mater. Interfaces, 2015, 7, 24622–24628 CAS.
- G. A. Ferrero, A. B. Fuertes and M. Sevilla, Electrochim. Acta, 2015, 168, 320–329 CrossRef CAS.
- J. R. Zhang, X. Wang, G. C. Qi, B. H. Li, Z. H. Song, H. B. Jiang, X. H. Zhang and J. L. Qiao, Carbon, 2016, 96, 864–870 CrossRef CAS.
- J. P. Han, G. Y. Xu, H. Dou and D. R. MacFarlane, Chem.–Eur. J., 2015, 21, 2310–2314 CrossRef CAS PubMed.
- J. C. Zhang, J. S. Zhou, D. Wang, L. Hou and F. M. Gao, Electrochim. Acta, 2016, 191, 933–939 CrossRef CAS.
- J. G. Jiang, H. Chen, Z. Wang, L. K. Bao, Y. W. Qiang, S. Y. Guan and J. D. Chen, J. Colloid Interface Sci., 2015, 452, 54–61 CrossRef CAS PubMed.
- B. Hu, K. Wang, L. H. Wu, S. H. Yu, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 813–828 CrossRef CAS PubMed.
- Z. Q. Wang, L. X. Sun, F. Xu, X. J. Peng, Y. J. Zou, H. L. Chu, L. Z. Ouyang and M. Zhu, RSC Adv., 2016, 6, 1422–1427 RSC.
- J. Górka, A. Zawislak, J. Choma and M. Jaroniec, Carbon, 2008, 46, 1159–1174 CrossRef.
- F. B. Su, C. K. Poh, J. S. Chen, G. W. Xu, D. Wang, Q. Li, J. Y. Lin and X. W. Lou, Energy Environ. Sci., 2011, 4, 717–724 CAS.
- L. Wei, M. Sevilla, A. B. Fuertes, R. Mokaya and G. Yushin, Adv. Funct. Mater., 2012, 22, 827–834 CrossRef CAS.
- J. Choma, K. Jedynak, W. Fahrenholz, J. Ludwinowicz and M. Jaronie, Appl. Surf. Sci., 2014, 289, 592–600 CrossRef CAS.
- J. Górka, A. Zawislak, J. Choma and M. Jaroniec, Appl. Surf. Sci., 2010, 256, 5187–5190 CrossRef.
- H. S. Teng and S. C. Wang, Carbon, 2000, 38, 817–824 CrossRef CAS.
- M. Chen, L. L. Shao, Y. P. Liu, T. Z. Ren and Z. Y. Yuan, J. Power Sources, 2015, 283, 305–313 CrossRef CAS.
- E. R. Pinero, D. C. Amorós and A. L. Solano, Carbon, 2003, 41, 1925–1932 CrossRef.
- H. Y. Liu, H. H. Song, X. H. Chen, S. Zhang, J. S. Zhou and Z. K. Ma, J. Power Sources, 2015, 285, 303–309 CrossRef CAS.
- K. Wang, N. Zhao, S. W. Lei, R. Yan, X. D. Tian, J. Z. Wang, Y. Song, D. F. Xu, Q. G. Guo and L. Liu, Electrochim. Acta, 2015, 166, 1–11 CrossRef CAS.
- Y. Fang, B. Luo, Y. Jia, X. Li, B. Wang, Q. Song, F. Kang and L. Zhi, Adv. Mater., 2012, 24, 6348–6355 CrossRef CAS PubMed.
- X. L. Yu, J. M. Lu, C. Z. Zhan, R. T. Lv, Q. H. Liang, Z. H. Huang, W. Shen and F. Y. Kang, Electrochim. Acta, 2015, 182, 908–916 CrossRef CAS.
- F. W. Ma, H. Zhao, L. P. Sun, Q. Li, L. H. Huo, T. Xia, S. Gao, G. S. Pang, Z. Shi and S. H. Feng, J. Mater. Chem., 2012, 22, 13464–13468 RSC.
- T. Y. Wei, X. L. Wei, Y. Gao and H. M. Li, Electrochim. Acta, 2015, 169, 186–194 CrossRef CAS.
- W. W. Liu, X. B. Yan, J. W. Lang, J. T. Chen and Q. J. Xue, Electrochim. Acta, 2012, 60, 41–49 CrossRef CAS.
- M. Zhou, F. Pu, Z. Wang and S. Y. Guan, Carbon, 2014, 68, 185–194 CrossRef CAS.
- A. B. Fuertes, F. Pico and J. M. Rojo, J. Power Sources, 2004, 133, 329–336 CrossRef CAS.
- G. H. Sun, J. Wang, K. X. Li, Y. Q. Li and L. J. Xie, Electrochim. Acta, 2012, 59, 424–428 CrossRef CAS.
- Y. Wang, R. Yang, M. Li and Z. J. Zhao, Ind. Crops Prod., 2015, 65, 216–226 CrossRef CAS.
- X. M. Ma, L. H. Gan, M. X. Liu, P. K. Tripathi, Y. H. Zhao, Z. J. Xu, D. Z. Zhu and L. W. Chen, J. Mater. Chem. A, 2014, 2, 8407–8415 CAS.
- X. M. Ma, M. X. Liu, L. H. Gan, Y. H. Zhao and L. W. Chen, J. Solid State Electrochem., 2013, 17, 2293–2301 CrossRef CAS.
- S. J. Su, Q. X. Lai and Y. Y. Liang, RSC Adv., 2015, 5, 60956–60961 RSC.
- T. X. Shang, R. Q. Ren, Y. M. Zhu and X. J. Jin, Electrochim. Acta, 2015, 163, 32–40 CrossRef CAS.
- C. X. Guo, N. Li, L. L. Ji, Y. W. Li, X. M. Yang, Y. Lu and Y. F. Tu, J. Power Sources, 2014, 247, 660–666 CrossRef CAS.
- B. G. Choi, M. H. Yang, W. H. Hong, J. W. Choi and Y. S. Huh, ACS Nano, 2012, 6, 4020–4028 CrossRef CAS PubMed.
- S. J. He, H. Q. Hou and W. Chen, J. Power Sources, 2015, 280, 678–686 CrossRef CAS.
- H. Y. Liu, H. H. Song, X. H. Chen, S. Zhang, J. S. Zhou and Z. K. Ma, J. Power Sources, 2015, 285, 303–309 CrossRef CAS.
- Y. Li, C. X. Lu, S. C. Zhang, F. Y. Su, W. Z. Shen, P. C. Zhou and C. L. Ma, J. Mater. Chem. A, 2015, 3, 14817–14825 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: CV curves of NO-MMCMs-700 and NO-MMCMs-850 at different scan rates ranging from 5 to 100 mV s−1; relative percentage of nitrogen and oxygen species obtained from deconvoluted XPS spectra. See DOI: 10.1039/c6ra16608c |
| This journal is © The Royal Society of Chemistry 2016 |