DOI:
10.1039/C6RA16567B
(Paper)
RSC Adv., 2016,
6, 93842-93854
Tuned synthesis and characterizational insight into β-cyclodextrin amended hydrous iron-zirconium hybrid oxide: a promising scavenger of fluoride in aqueous solution†
Received
27th June 2016
, Accepted 16th September 2016
First published on 16th September 2016
Abstract
The consumption of water contaminated with fluoride (>1.5 mg L−1) causes serious problems to public health and ultimately leads to skeletal fluorosis. Thus, the development of more efficient fluoride scavenging materials for designing water filters is an immediate task for researchers. β-Cyclodextrin (β-CD) amended hydrous iron–zirconium hybrid oxide (CHIZO), which is a new type of surface modified highly selective composite in organic–inorganic frameworks, is synthesized and characterized using various state of the art analytical tools, and its efficacy on fluoride removal from an aqueous solution is explored. The agglomerated micro structured composite material has no significant fingerprint such as surface appearance in TEM images and is inclined to possess very poor crystallinity. The BET analysis of CHIZO reveals a surface area of 0.2070 m2 g−1 and pore volume of 0.0476 cm3 g−1. The highly pH dependent fluoride adsorption by CHIZO decreases with an increase in pH, and pseudo-second order kinetics control the reaction. The Langmuir isotherm was recognized to be the best fit model to describe the adsorption equilibrium with a significantly higher monolayer adsorption capacity of fluoride (31.35 mg g−1) than the host hydrous Fe–Zr oxide (8.21 mg g−1) at pH ∼7.0 and 303 K. The thermodynamically spontaneous nature of CHIZO is due to the exothermic nature of the reaction. In addition, phosphate and sulphate show an adverse effect on fluoride adsorption. β-CD forms inclusion complexes by taking up fluoride ions from water into its central cavity and the driving forces associated with the complex formation include the release of enthalpy-rich water molecules from its cavity, electrostatic interactions, hydrogen bonding and release of conformational strain. The poor regeneration of the spent adsorbent even in 1.0 M NaOH (below 20%) is probably a consequence of entrapping fluoride inside the cavity of β-CD with hydrogen bonding. It has been found that only 0.9 g of CHIZO is able to reduce the fluoride level to below 1.0 mg L−1 in one-litre of fluoride spiked (5.0 mg L−1) natural water sample. The present study thus reveals that CHIZO could be an efficient adsorbent for fluoride because of its high adsorption capacity and economical viability.
1 Introduction
Vast parts of the world have fallen prey to the two-pronged threats of arsenic and fluoride contamination, which are major contributors to water contamination in the globe. Along with arsenic contamination, fluoride is fast assuming menacing proportions in vitiating drinking water sources around the world since its discovery in the Nellore district of Andhra Pradesh (India) in 1937.1 Spiked levels of fluoride in aquifers mostly result from its mobilization in natural conditions from fluoride bearing minerals. The recommended value of optimum fluoride levels in drinking water is fixed at 1.5 mg L−1 by the World Health Organization (WHO),2 which is often not encountered in groundwater systems, thus leading to dental and bone fluorosis.3 Groundwater constitutes 97% of global fresh water resources and forms a single point source for providing drinking water, and for most communities it may be the only economically viable option for this purpose. Thus, access to clean groundwater is of paramount importance, since it is critical in different spheres of human habitations. Excessive groundwater abstraction in relation to recharge has led to the depletion of this resource and impairs the drinking water supply. Currently, worldwide groundwater quality is suffering due to widespread fluoride contamination which has struck twenty-five nations and more than 200 million people around the world.4 Over the past seven decades, the prevalence and severity of fluorosis have increased in leaps and bounds in India, reaching almost epidemic proportions, and as such today at least 18 states in India are suffering from fluorosis. In fact fluoride contamination today is arguably even more widespread than the arsenic problem, which reiterates its growing significance. Also, it has become a socio-economic issue rather than merely a health issue. The right to safe water still remains an unfulfilled dream today and this necessitates extensive research on earth-friendly remediation techniques, especially in the Indian context, to provide a holistic solution to the fluoride menace. Economic viability is critical to any defluoridation solution, since a major part of rural India is dependent on groundwater. A few viable techniques for defluoridation have been explored by Jagtap et al.,5 among which adsorption appears to be feasible due to its dual advantage of being cost-effective and highly efficient. Among the various usable adsorbents, synthetic hydrous elemental oxides in various forms (including hybrid metal oxides) have emerged as potent defluoridation options over the years.6–12 Modification of these oxides has also yielded promising results and much of the global research is now veering towards deploying modified materials toward contaminant removal.13 β-Cyclodextrin (β-CD), which is an inexpensive, sustainably produced macrocycle of glucose, is of interest for the removal of micro pollutants from water by means of adsorption and encapsulation to form well-defined host–guest complexes, and enhancing the adsorption of organic/inorganic pollutants.14–21 Due to their torus-like geometry, relatively hydrophobic surface of their internal cavity and hydrophilic character of their external hydroxyl groups, β-CD molecules easily form inclusion complexes with a wide variety of organic and inorganic molecules. β-CD has the lowest water solubility (18 g L−1) of all the CDs due to the formation of intramolecular H-bonds among the hydroxyl groups in its external cavity.
It can also be a macrocyclic container by crowning on the surface of metal oxides/hydroxides, which may subsequently enhance the adsorption of organic/inorganic pollutants.22 This hypothesis is put into use, whereby an iron–zirconium hybrid oxide (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio) surface is modified in an optimized fashion with β-CD. The molecule has several –OH groups which may anchor metal oxides with the generation of a metal oxide–β-CD composite and this feature can be used to entrap and remove contaminants from aqueous solution through the formation of inclusion complexes with β-CD.23–26
1 mole ratio) surface is modified in an optimized fashion with β-CD. The molecule has several –OH groups which may anchor metal oxides with the generation of a metal oxide–β-CD composite and this feature can be used to entrap and remove contaminants from aqueous solution through the formation of inclusion complexes with β-CD.23–26
Herein, we report the characterization of the as-prepared composite material and compare it with the unmodified material, and exploration of the efficacy of the synthetic composite materials for the removal of fluoride from aqueous solution.
2 Materials and methods
2.1 Chemicals
Anhydrous sodium fluoride (NaF), sodium-2-(para-sulfophenyl azo)-1,8-dihydroxy-3,6-naphthalene disulfonate (SPADNS), zirconium oxychloride octahydrate (ZrOCl2·8H2O), ferric chloride (FeCl3), sodium chloride (NaCl) and β-cyclodextrin (β-CD) were obtained from E. Merck (India), Loba Chemie (India) and BDH (England).
2.2 Fluoride solution
A stock solution of fluoride (1000 mg L−1) was prepared by dissolving 2.210 g of NaF in 1.0 L of double de-ionized water. The working solution of desired concentrations were made by exact dilution with 0.01 M NaCl solution (ionic strength, I = 0.01 M). The initial concentrations of fluoride solution, [F]0 used for the experiments were in the range of 5.0 and 35 mg L−1 at an I = 0.01 M.
2.3 Synthesis of β-cyclodextrin modified hydrous iron–zirconium hybrid oxide (CHIZO)
The synthetic pathway employed for CHIZO is similar to the method reported for the preparation of β-CD modified hydrous ferric oxide.13 Hydrous iron–zirconium hybrid oxide (HIZO) was synthesized by dissolving ferric chloride and zirconium oxy-chloride in 0.1 M HCl, separately, and mixing them in a wide range of pre-determined ratios (v/v) followed by neutralization using a 2.1 M NH4OH solution with constant stirring until the pH of supernatant reached ∼7.0. The co-precipitated brown mass was aged for 48 hours, filtered, washed with distilled water until it was free from chloride, followed by drying at 100 °C and finally crushed into agglomerated particles of size 140–290 μm (52–100 mesh) prior to use for all experiments. For the preparation of β-CD modified hydrous iron–zirconium hybrid oxide (CHIZO), various quantities of β-CD solutions were mixed with the preselected molar ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of FeCl3 and ZrOCl2 admixture followed by adjustment of the pH to ∼7.0, aging, filtration, drying and sieving as before. The fabricated host–guest complex between the β-CD and HIZO composites was formed using the above procedure. The synthesized materials were optimized for the removal efficiency of fluoride and the maximum efficiency was achieved for the molar composition of β-CD
1 of FeCl3 and ZrOCl2 admixture followed by adjustment of the pH to ∼7.0, aging, filtration, drying and sieving as before. The fabricated host–guest complex between the β-CD and HIZO composites was formed using the above procedure. The synthesized materials were optimized for the removal efficiency of fluoride and the maximum efficiency was achieved for the molar composition of β-CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr as 0.2
Zr as 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Tables 1 and 2).
1 (Tables 1 and 2).
Table 1 Fe/Zr mole proportion optimization for fluoride uptake from aqueous solution at pH 7.0 and 303 Ka
| Fe/Zr mole ratio |
qF (mg g−1) (C0 = 5.0 mg L−1) |
qF (mg g−1) (C0 = 10.0 mg L−1) |
qF (mg g−1) (C0 = 20.0 mg L−1) |
| qF: fluoride adsorption capacity (mg g−1). |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1 |
9.4 |
18.1 |
20.4 |
9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
6.1 |
17.8 |
13.8 |
8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
7.7 |
8.1 |
15 |
9.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
6.4 |
9.7 |
16.2 |
8.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
8.7 |
10.8 |
15 |
Table 2 Mole proportion of β-CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr optimization for fluoride adsorption at pH 7.0 from watera
Zr optimization for fluoride adsorption at pH 7.0 from watera
Mole ratio of β-CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr Zr |
qF (mg g−1) |
| qF: fluoride adsorption capacity (mg g−1). |
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.68 |
0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4.57 |
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1 |
7.8 |
0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.61 |
2.4 Fluoride analysis
Fluoride samples were analyzed using a spectrophotometer (Model U-4100, Hitachi, Japan) by adopting the standardized procedure as described in the “Standard Methods for the Examination of Water and Wastewater”.27 A 50 mL sample of fluoride concentration,[F0], ranging from 0 to 1.4 mg L−1 was placed in a 200 mL polyethylene flask followed by the addition of 10 mL reagent containing equal volumes of SPADNS and zirconyl acid reagent (prepared by dissolving ZrOCl2 in deionized water along with concentrated HCl) and mixed well. The spectrophotometer was set to zero absorbance with the reference solution. To prepare the reference solution 10 mL SPADNS solution was added to 100 mL distilled water, and 7 mL concentrated HCl was diluted to 10 mL with distilled water and added to the diluted SPADNS solution. The resulting solution was used for the reference set, and the absorbance of the working solutions was measured at 570 nm. A freshly prepared reference was used for each set of measurements. The fluoride concentration was estimated from the calibration curve.
2.5 Analytical tools
Powder X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM), Brunauer–Emmett–Teller (BET) nitrogen (vapour) adsorption–desorption and Fourier transform infrared (FTIR) spectroscopy were used to characterize the materials. X-ray diffraction (XRD) patterns of the material were recorded using an X-ray powder diffractometer (Philips Analytical PW-1710) equipped with Cu-Kα radiation (λ = 1.5418 Å) at a scanning speed 0.4° per min between the angle 10° and 70°, operated at the voltage of 40 kV and applied potential current of 30 mA. Transmission electron microscopic (TEM) images were recorded on an H800 transmission electron micrograph operated at 200 kV. The samples for the TEM were dispersed in isopropanol by sonication, and drops were casted onto 200 mesh copper grids coated with a porous carbon film. Atomic force microscopic (AFM) images were taken by an Innova Atomic Force Microscope (Bruker AXS Pte Ltd) using optical beam deflection to monitor the displacement of a micro-fabricated silicon cantilever with a spring constant of 80 N m−1 to visualize the topography of the composite surface. The surface area of the materials were analyzed by the Brunauer–Emmett–Teller (BET) method via N2(vapour) adsorption at 77 K using a high-speed surface analyzer (model: ASAP 2000, Norcross, USA). The adsorption of N2(vapour) at a temperature of 77 K leads to an adsorption isotherm commonly referred to as the Brunauer–Emmett–Teller (BET) isotherm. Multiplying the number of gas molecules required to cover an adsorbent surface with a monolayer of adsorbed molecules with the cross-sectional area of the adsorbate material gives the approximate sample surface area. The pHPZC value of the samples was analyzed via the pH metric method used by Babic et al.28
2.6 Experimental
2.6.1 Batch adsorption experiment. Adsorption reactions by batch experiments were conducted by the addition of 25 mg CHIZO (or HIZO) to 50 mL of 10 mg L−1 fluoride solution (I = 0.01 M) in a series of 200 mL polythene (PE) bottles. The desired pH of each solution was adjusted with 0.1 M HCl and/or NaOH, and agitated (300 ± 10 rpm) at 303 K for 120 minutes. Kinetic experiments were also conducted as above employing a fluoride solution of [F−]0 = 10 mg L−1 (at I = 0.01 M), and separate measurements at pH 3.0 (<pHPZC of CHIZO) and 7.0 (>pHPZC of CHIZO) at 303 K. Isotherm experiments for the fluoride adsorption over CHIZO were also identical, with [F−]0 in the range of 5.0–35.0 mg L−1 at I = 0.01 M, and conducted at pH 7.0 (around the pH of drinking water) at 303, 318 and 333 K, separately. Fluoride adsorption experiments were conducted as above in the presence of phosphate (0.0–3.0 mg L−1), sulphate (0.0–50.0 mg L−1), nitrate (0.0–50.0 mg L−1) and chloride (0.0–100.0 mg L−1). The pH (pHi) of the suspension was recorded in each stage by a pH-meter (model LI-127, ELICO, India). Once the agitation time was complete, the suspensions were filtered immediately using 0.45 μm membrane filters, and the filtrates were analyzed for fluoride27 by a UV-VIS spectrophotometer (Hitachi model U-3210, Japan). The fluoride adsorption capacity was calculated using the relation [(Ci − Cf)V]/w, where Ci and Cf are the initial and final fluoride concentrations (mg L−1), respectively, V is the volume (L) of test solution, and w is the mass (g) of the adsorbent.
3 Results and discussion
3.1 Characterization of β-cyclodextrin modified hydrous iron–zirconium hybrid oxide (CHIZO)
The synthetic HIZO and CHIZO materials prepared using the methods described earlier (Sub-section 2.3) were fully characterized. The crystalline structure and phase of the composite materials were determined via the powder X-ray diffraction (XRD) technique using a Philips Analytical PW-1710X-ray powder diffractometer with the Bragg–Brentano goniometer geometry and Cu-Kα X-ray radiation source (λ = 1.5418 Å). Fig. 1 shows the XRD patterns obtained for the crystalline structure and phase of the composite (surface modified) and unmodified materials. Compositions, as well as purity, were examined by the X-ray diffraction technique and the resulting diffractrograms are displayed in Fig. 1A–C. The broadness of the diffraction peaks indicates the formation of particles with very small dimensions of composite crystallites. The sharp peaks with relatively narrow FWHM (Full Width Half Maxima) indicate high crystallinity due to presence of composite Fe–Zr oxide in Fig. 1A, whereas the incorporation of β-CD reduces the intensity of the characteristic M–O peaks as a consequence of the changes in phase from crystalline to amorphous (Fig. 1B). A gradual loss of crystallinity and intensity of the CHIZO composite is observed with an increase in the concentration of β-CD.
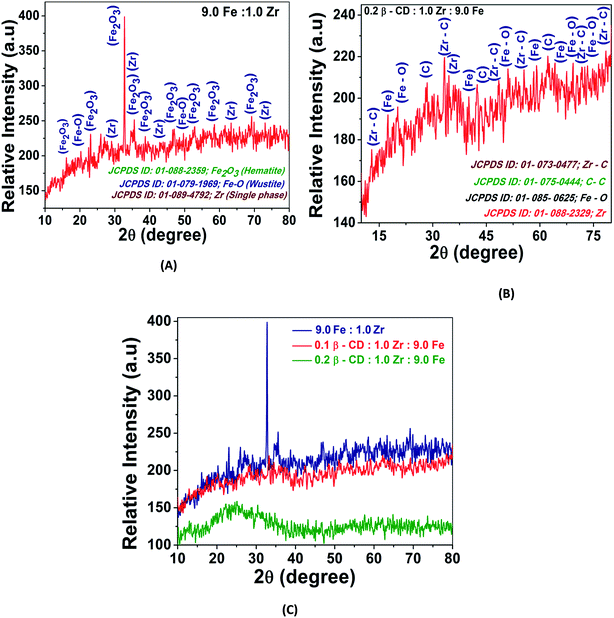 |
| | Fig. 1 X-ray diffraction patterns of the Fe–Zr mixed oxide (A), β-CD modified Fe–Zr mixed oxide (B) and comparison of the XRD patterns of variable contents of β-CD in the Fe–Zr mixed oxide (C). | |
Fig. 2 shows the SEM images with EDX spectra (inset) of HIZO, CHIZO and fluoride adsorbed CHIZO, which show the morphological change and atomic weight percentage distribution of the various constituents of HIZO, CHIZO and fluoride adsorbed CHIZO. It is evident that the surfaces possess an irregular morphology and agglomeration. However, the surface smoothness increased from image-A (HIZO) to image-B (CHIZO) and reduced from image-B to image-C (fluoride adsorbed CHIZO). It also is evident that the surfaces possess appreciable porosity and agglomeration. The surface composition analysis of the EDX spectrum also reveals the presence of carbon, which supports the surface modification of HIZO with β-CD.
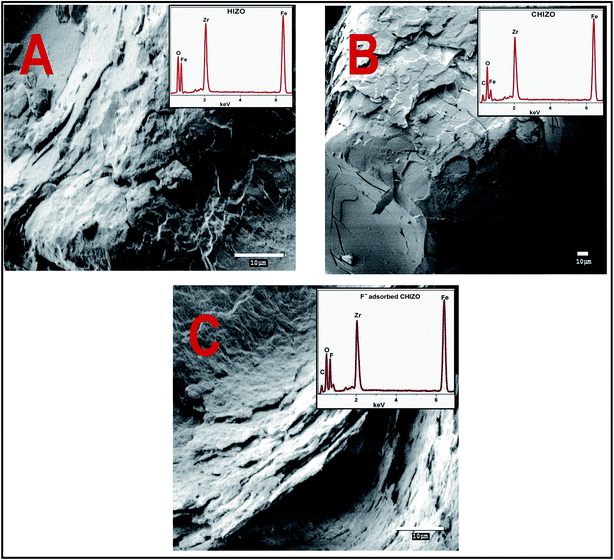 |
| | Fig. 2 SEM images including the EDX spectra of HZO (image-A), CHIZO (image-B), and fluoride adsorbed CHIZO (image-C). | |
Fig. 3 shows TEM images of HIZO (images A & C) and CHIZO (images B & D) at two different magnifications. Fig. 3A shows the presence of agglomerated particles in HIZO, however the degree of agglomeration in CHIZO is much higher, as shown in the TEM image (B & D in Fig. 3), in which no individual particles are observed. The particle size of HIZO is ∼6–8 nm, as estimated from the TEM image-C of Fig. 3. The fingerprint like signature in the TEM image of HIZO (C in Fig. 3) indicates the presence of crystalline planes, hence the crystalline nature of the substance. TEM image-D shows no signature of crystalline planes (Fig. 3), which indicates the amorphous nature of CHIZO. This is an indicator of the heightened adsorption capability of CHIZO in comparison to HIZO.
 |
| | Fig. 3 TEM images at two different magnifications of HIZO (A and C) and CHIZO (B and D). | |
Fig. 4 shows the atomic force microscopic (AFM) images of HIZO (image-A), and CHIZO (image-B) and fluoride adsorbed CHIZO (image-C) in 2D and 3D. The 3-D views of the AFM images of CHIZO and HIZO manifest the higher degree of surface roughness of the former than the latter material. It can be seen that the horizontal distance in CHIZO is larger than in HIZO. This is probably due to the sharpening of peaks in CHIZO compared in HIZO, which leads to an elongation of the peaks and in turn heightens the roughness of the CHIZO sample. This increased roughness of CHIZO compared to HIZO is not surprising since the β-CD moieties hinge on to the HIZO surface in CHIZO through H-bonding, which naturally wrinkles the surface to a large extent. However, the decrease in wrinkled nature (image-C) of the fluoride adsorbed material is evident with surface coverage by fluoride on CHIZO.
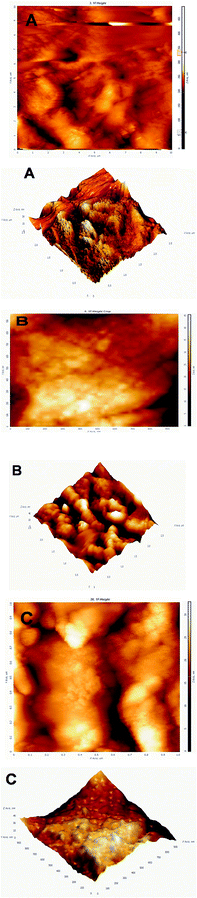 |
| | Fig. 4 AFM images of HIZO (A), CHIZO (B) and F-adsorbed CHIZO (C) for surface morphological patterns in 2-D and 3-D views. | |
Fig. 5 shows the FTIR spectra of HIZO and β-CD encapsulated HIZO (CHIZO). The FTIR bands at 3200, 1622, 1440 and 680 cm−1 of HIZO may be assigned to the –OH stretching, bending, carbonate and Fe–O–Zr bond vibration, respectively. When Fe–Zr binary oxide forms an inclusion complex with β-CD, a different FTIR spectrum is obtained. This FTIR spectrum shows –OH stretching and bending mode vibration at 3250 and 1620 cm−1, respectively. The bands at 1158, 2900, 1082 and 1030 cm−1 correspond to C–C, –CH2, C–O ether and C–OH of β-CD, respectively. The characteristic bands of the Fe–O–Zr bond are diminished, as shown in the FTIR spectrum, which clearly indicate that the oxide must undergo encapsulation with β-CD.
 |
| | Fig. 5 FTIR spectra of HIZO, CHIZO and F− adsorbed CHIZO. | |
Fig. 6 shows the BET isotherm plots for CHIZO, HIZO and fluoride incorporated CHIZO. The immense difference in the surface area and pore volumes between CHIZO and HIZO (∼0.2070 m2 g−1 and 0.0476 cm3 g−1; 3.0911 m2 g−1 and 0.7101 cm3 g−1) confirms the surface modification of HIZO with β-CD and this in turn is responsible for the proliferation of surface active –OH groups on CHIZO. This also explains the increase in adsorption capacity of CHIZO in relation to HIZO despite a fall in surface area, which is an observation in accord with the observations in our previous studies.13 The decreased pore volume of CHIZO compared to HIZO results from the inhibition of N2 molecules to access the binding sites due to the adherence of β-CD on the inorganic surface which in turn sharply diminishes the surface area of CHIZO. The BET surface area of 0.1994 m2 g−1 and pore volume of 0.0458 cm3 g−1 for the fluoride impregnated CHIZO are consistent with the observed surface area of CHIZO.
 |
| | Fig. 6 Plots of N2 (vapour) (cm3 g−1) adsorbed and desorbed against relative pressure for the BET isotherm plots of CHIZO (A), HIZO (B) and fluoride incorporated CHIZO (C). | |
The pHPZC of HIZO and CHIZO was analyzed, which was found to be 5.1 and 4.5, respectively. Fig. 7 shows the shifting of pHPZC for CHIZO to a more acidic zone, which is attributed to the acidic –OH functional groups of the incorporated β-CD on HIZO. The hybrid metal oxide–β-CD composite can develop positive, neutral or negative surface charge, depending upon the ambient solution pH, through the abstraction or release of H+ ions at or from the active hydroxyl groups. The pHPZC, which is the pH value of zero surface charge, of CHIZO was found to be shifted to the lower side of the acid pH range compared to HIZO. This is probably as a consequence of the substitution of surface water molecules of hydrous metal oxides by β-CD, thus indicating an abundance of surface active –OH groups on CHIZO compared to HIZO.29
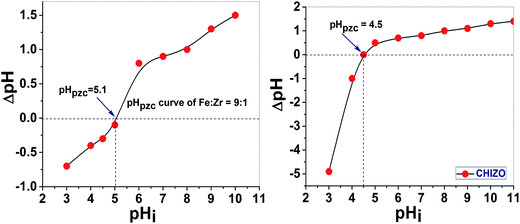 |
| | Fig. 7 Plots of ΔpH versus pHi for the estimation of pHZPC of HIZO and CHIZO. | |
3.2 Effect of pH
The effect of pH on the removal of fluoride was determined in an aqueous fluoride solution (10 mg L−1) using an adsorbent dose of 0.5 g L−1 at 303 K and a broad range of pH (3–10). Fig. 8 shows the results of the percentages of fluoride adsorption by HIZO and CHIZO. It has been found that there is a sharp decrease in the percentage of fluoride removed with an increase in pH, and this trend was more profound in the case of CHIZO. The percentage of fluoride adsorption by CHIZO was ∼78, ∼50 and ∼35, while by HIZO it was ∼45, ∼40 and ∼15 at pH 3, 5 and 10, respectively. It can be seen that the with an increase in pH from ∼3 to 4.5 (pH < pHPZC < 5), the percentage of fluoride adsorption decreases steadily as a consequence of the diminishing electrostatic attraction between the gradually reduced positive charged sites of the adsorbent and negatively charged fluoride ions. At pH > pHPZC, the surface of the adsorbent becomes more negative and experiences enhanced electrostatic repulsion with the anionic fluoride ion, and subsequently fluoride adsorption is reduced significantly. At lower pH, especially below the pHPZC, the positive charge over the solid surface accelerates the adhesion of fluoride ions from solution to the solid surface owing to columbic forces, which explains the upward trend in adsorption capacity. It is evident that CHIZO is a more efficient scavenger of fluoride than HIZO in acidic pH, and this agrees well with the results by other researchers who observed enhanced adsorption of fluoride with a decrease in pH by different adsorbents.30–34
 |
| | Fig. 8 Plots of the percentage of fluoride adsorption versus pH over the surfaces of CHIZO and HIZO, separately, at 303 K from a fluoride solution (Ci = 10 mg L−1). | |
3.3 Adsorption kinetics
Fig. 9 demonstrates the kinetic data (as points) of the fluoride adsorption reaction with CHIZO from an aqueous solution of fluoride, with the concentration of 10 mg L−1 at 303 K and two separate pH values below pHPZC (3.0) and above pHPZC (7.0). Modeling of the kinetic data (Fig. 9) by the non-linear least square fit method was done separately with the pseudo-first order (eqn (1)) and pseudo-second order (eqn (2)) equations.35| | |
qt = t × k2 × qe2/[1 + (t × k2 × qe)]
| (2) |
where, k1 (min−1), k2 (g mg−1 min−1), qt and qe represent the pseudo-first order rate constant, the pseudo-second order rate constant, amount of fluoride adsorbed (mg g−1) at any time t (min) and at equilibrium, respectively. The modeled parameters of eqn (1) and (2) estimated from the plots are represented in Table 3. The kinetic model that agrees better with the kinetic data is judged based on the respective regression coefficient (R2) and agreement between the experimental and calculated value of qe. The experimental adsorption capacities, (qt)exp were found to be closer to the modeled adsorption capacities, (qt)model of pseudo-second order kinetics than pseudo-first order, which indicate that the kinetics of the adsorption reaction obey the assumptions of the pseudo-second order model. Fig. 9 reveals that about 80% of the (qt)exp value of fluoride takes place in one-third fraction of the equilibrium time (∼2 h). The initial fast adsorption is due to the rapid diffusion of solute through the boundary surface of the liquid–solid interface as a consequence of electrostatic interaction, while the later slow adsorption is presumably due to the enhanced columbic repulsion exerted between the adsorbed species and the adsorbate remaining in solution, which does not allow the solute to cross over the diffusion barrier at the solid–liquid boundary layer. It is seen that the regression coefficients, R2 (Table 3), at 303 K were found to be 0.68 and 0.72 for the pseudo-first order reaction, and 0.97 and 0.98 for the pseudo-second order reaction for 10 mg L−1 fluoride at pH 3 and 7, respectively, which suggest that pseudo-second order is the best fit kinetic model. The uptake of fluoride by different solid matrices from the aqueous stream through the pseudo-second order kinetic model has also been reported by various researchers.6,10,13,30,31,36,37
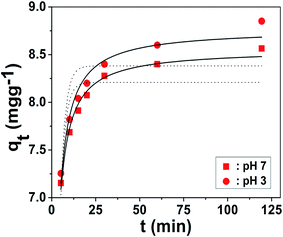 |
| | Fig. 9 Plots of fluoride adsorption amount (qt, mg g−1) by CHIZO against time (t, min) at 303 K from a fluoride solution of concentration 10 mg L−1 (ionic strength = 1 M) at pH 3.0 and pH 7.0. | |
Table 3 Kinetic model parameters of fluoride adsorption over CHIZO surface at the temperature of 303 K and two different pH valuesa
| Concentration ([Fo], mg L−1) |
Adsorbent |
Pseudo-first order qt = qe[1 − exp(−k1t)] |
Pseudo-second order qt = tk2qe2/(1 + k2qet) |
| k1 |
qe |
R2 |
χ2 |
k2 |
qe |
R2 |
χ2 |
| qe (mg g−1), k1 (min−1), k2 (g mg−1 min−1). |
| 10 |
CHIZO (pH = 3.0) |
0.364 |
8.38 |
0.68 |
0.107 |
0.0977 |
8.768 |
0.97 |
0.0115 |
| CHIZO (pH = 7.0) |
0.374 |
8.21 |
0.72 |
0.077 |
0.1087 |
8.556 |
0.98 |
0.0043 |
3.4 Equilibrium modeling
Since adsorption isotherms relate the amount of adsorbate attached on the surface of an adsorbent with equilibrium concentration at a definite temperature, the equilibrium data were analyzed by Langmuir and Freundlich isotherm models to understand the adsorption mechanism. The Langmuir isotherm equation (eqn (3)) was developed based on the assumption that the adsorption sites are homogeneous and adsorption takes place with monolayer surface coverage with adsorbate, whereas the Freundlich isotherm equation (eqn (4)) was developed based on multilayer surface coverage with adsorbate and heterogeneity of adsorption sites.35–38 These two equations are as follows:| | |
qe = qm × KL × Ce(1 + KL × Ce)
| (3) |
where, qm is the maximum monolayer adsorption capacity (mg g−1) and KL is the equilibrium constant related to adsorption energy (L mg−1).39 The KF is the Freundlich adsorption capacity and 1/n is an arbitrary constant related to the adsorption intensity. The other terms, such as Ce and qe, have their usual significance. The equilibrium data shown in Fig. 10 were analyzed by the non-linear fit method with the Langmuir (eqn (3)) and Freundlich (eqn (4)) models. The estimated parameters related to the above equations are given in Table 4 including regression coefficient (R2) and statistical error chi-square (χ2). It is evident that the equilibrium data were fitted well by the Langmuir model (eqn (3)). Moreover, the values of qm obtained from the isotherm model were almost identical with the experimental values, which suggest that the adsorption isotherm follows the Langmuir model well. The Langmuir monolayer adsorption capacity, qm, was found to be 44.9, 31.4 and 10.6 mg g−1 at 288, 303 and 318 K, respectively. The decrease in fluoride adsorption by CHIZO with an increase in reaction temperature indicates that the adsorption is exothermic in nature. The estimated qm for CHIZO was found to be higher than the other adsorbents (Table 5) reported in the literature.8–11,38,40,41 The present study indicates that CHIZO could be an efficient material for scavenging fluoride from contaminated water.
 |
| | Fig. 10 Plots of fluoride adsorption amount (qe, mg g−1) against equilibrium fluoride concentration (Ce, mg L−1) of CHIZO at different temperatures (288, 303 and 318 K) and at pH 7.0 (— Langmuir isotherm fit; ⋯ Freundlich isotherm fit). | |
Table 4 Isotherm model parameters evaluated for fluoride adsorption on CHIZO (pH = 7) at the temperatures of 288, 303 and 318 K
| Isotherm models |
Parameters |
288 K |
303 K |
318 K |
| Langmuir isotherm: qe = qmKLCe/(1 + KaCe) |
R2 |
0.96 |
0.97 |
0.95 |
| χ2 |
2.38 |
1.12 |
0.13 |
| qm |
44.9 |
31.35 |
10.6 |
| Ka |
0.054 |
0.05 |
0.28 |
| Freundlich isotherm: qe = KFCe1/n |
R2 |
0.93 |
0.93 |
0.89 |
| χ2 |
3.66 |
2.41 |
0.32 |
| KF |
2.89 |
2.69 |
4.1 |
| n |
1.55 |
1.68 |
3.79 |
Table 5 Comparison of Langmuir adsorption capacity of CHIZO with fluoride at T = 303 K with some literature values
| Adsorbent material |
pH |
Langmuir adsorption capacity, (mg g−1) |
Reference |
| Hydrous ferric oxide |
6.8 ± 0.1 |
7.50 |
8 |
| Fe3O4@Al(OH)3 magnetic nanoparticles |
6.5 |
88.48 |
33 |
| Bauxite |
7.0 |
5.16 |
34 |
| Fe(III)–Zr(IV) hybrid oxide |
6.8 ± 0.1 |
8.21 |
10 |
| Fe(III)–Al(III) mixed oxide |
6.9 ± 02 |
17.73 |
11 |
| Fe(III)–Sn(IV) binary mixed oxide |
6.4 ± 0.2 |
10.47 |
12 |
| CHIZO |
7.0 |
31.35 |
Present work |
In addition, the equilibrium data was analyzed for the Dubinin–Radushkevich isotherm (ESI†), which showed that the energy adsorption (kJ mol−1) values are found to be 2.81 (at 288 K) and 2.64 (at 303 K). The energy values suggest that the fluoride adsorption is a physical phenomenon.
3.5 Thermodynamic parameters and energy of adsorption
Thermodynamic parameters, such as standard free energy (ΔG°), standard enthalpy (ΔH°) and standard entropy (ΔS°) changes, for this adsorption process were estimated using the following thermodynamic relations (eqn (7) and (8)), assuming the activity coefficient as unity at low solute concentrations.36and| |
ΔG° = −2.303RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc
| (6) |
From eqn (5) and (6), eqn (7) may be obtained as,
| |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc = ΔS°/2.303R − (ΔH°/2.303R)1/T Kc = ΔS°/2.303R − (ΔH°/2.303R)1/T
| (7) |
where, each term is represented by their usual significance. Substituting the thermodynamic equilibrium constant
Kc with
qe/
Ce,
eqn (7) is converted to
eqn (8).
| | |
log(qe/Ce) = ΔS°/2.303R − (ΔH°/2.303R)1/T
| (8) |
Assuming ΔS° and ΔH° to be constant within the range of studied temperature, the values were computed from the intercept and slope of the straight line of plots of log(qe/Ce) versus 1/T (Fig. 11), and ΔG° at various temperatures was estimated using eqn (6). The plots showed good linearity with very good regression coefficients (R2 = 0.99) by taking the fluoride concentration as 10 mg L−1, and the results are represented in Table 6. The ΔG° values for the fluoride adsorption by CHIZO indicate the thermodynamic feasibility/spontaneity of the reactions. The values of ΔG° obtained were −17.2636, −17.1091 and −16.9546 kJ mol−1 at 288, 303 and 318 K, respectively, which suggest that the adsorption of fluoride by CHIZO is spontaneous and favorable for a wide temperature range. The negative value of ΔH° (−20.23 kJ mol−1) reveals that the adsorption process is exothermic in nature. This fact is the same as that reported in the isotherm studies.41 The entropy change (ΔS°) was obtained as −0.0103 J mol−1 K−1, which indicates the irreversibility and appreciable stability of the adsorption process.
 |
| | Fig. 11 Plots of log(qe/Ce) versus T−1 (K−1) for the thermodynamic parameters for the fluoride adsorption reaction with CHIZO at 303 K. | |
Table 6 Thermodynamic parameters evaluated for fluoride adsorption reaction with CHIZO at pH = 7 and 25.0 mg F per L solution
| Species |
[F]0 (mg L−1) |
ΔH0 (kJ mol−1) |
ΔS0 (J mol−1 K−1) |
ΔG0 (kJ mol−1) |
| 288 K |
303 K |
318 K |
| CHIZO |
25 |
−20.23 |
−0.0103 |
−17.2636 |
−17.1091 |
−16.9546 |
3.6 Effect of co-occurring ions on fluoride adsorption
Among the anions commonly present in groundwater, phosphate and sulphate show an adverse influence on the fluoride adsorption over CHIZO from a solution of fluoride with a concentration of 10 mg L−1. With an increase in phosphate concentration from 0.0 to 3.0 mg L−1, the fluoride adsorption amount (8.56 mg g−1) is reduced to 4.4 mg g−1, which is about 50% of the initial value. However fluoride adsorption amount (8.56 mg g−1) is reduced to 6.15 mg g−1, which is about 28%, when the sulphate concentration was enhanced in solution from 0.0 to 50 mg L−1. Thus, the adverse effect of phosphate ions on fluoride adsorption over CHIZO is more prominent than sulphate. In contrast chloride (100 mg L−1) and nitrate (50 mg L−1) show no notable adverse influence on fluoride adsorption.
3.7 Adsorption mechanism
The surface of CHIZO is rich in –OH groups due to the presence of an encapsulated β-CD moiety. The tetragonal Zr4+ ions present inside the solid CHIZO composite have a strong affinity for fluoride and this driving force facilitates fluoride ions to enter into the wider end of the cylindrical β-CD cavity where negatively charged fluoride ions strongly adhere to the hydroxyl groups of β-CD through H-bonding, as shown in Fig. 12. Moreover, the small size of the fluoride ion facilitates the formation of hydrogen bonds with the –OH group of β-CD.42 The FTIR spectrum of the fluoride adsorbed CHIZO (Fig. 5) shows the –OH stretching mode of vibration at ∼3150 cm−1. This red shift in the FTIR spectrum during fluoride adsorption on CHIZO (which shows the –OH stretching vibration at 3250 cm−1) clearly supports the formation of H-bonds.43 Significantly the poor desorption of fluoride (<20% even with 1.0 M NaOH solution) from the fluoride adsorbed CHIZO achieved in this study supports the strong attachment of F− ions on the CHIZO surface through strong H-bonding. The mechanistic scheme of fluoride abstraction by the solid matrix from aqueous fluoride solution is suggested herein.
 |
| | Fig. 12 The mechanism of fluoride adsorption over the CHIZO surface at pH 7.0 and temperature of 303 K. | |
4 Fluoride removal from natural water
A natural water sample was taken from a hand pump attached tube-well (Colootola, College Street, Kolkata, India) and was analyzed for some physicochemical parameters, such as pH (7.36), Fe(total) (0.22 mg L−1), F− (0.45 mg L−1), HCO3− (550 mg L−1), Cl− (92 mg L−1), SO42− (65 mg L−1), Ca2+ (129 mg L−1), Mg2+ (58 mg L−1), hardness(total) (690 mg L−1), and TDS (1540 mg L−1). An appropriate quantity of fluoride solution was added to spike the fluoride level in this natural sample to 5.0 mg L−1. One liter of this water sample when agitated (500 rpm) for 2 hours with the help of magnetic stirring, which changed the dosage from 0.5 g to 1.5 g, showed a reduction in fluoride level from 5.0 mg L−1 to below 1.0 mg L−1 (below the WHO guideline maximum concentration level) with an increase in CHIZO dosage from 0.5 g to 0.9 g L−1. In addition, the analysed water quality parameters such as pH (6.8), Fe(total) (0.11 mg L−1), HCO3− (250 mg L−1), Cl− (52 mg L−1), SO42− (45 mg L−1), Ca2+ (101 mg L−1), Mg2+ (35 mg L−1), hardness(total) (460 mg L−1) and TDS (740 mg L−1) were also reduced compared to the pre-treated water, which suggest sufficient improvement of water quality.
5 Conclusion
CHIZO, which is a composite of HIZO and β-CD, was used for fluoride adsorption. It is an amorphous functional material with an irregular surface morphology and high surface site density. Its particle size on average is 150 nm, which is higher than pristine HIZO (∼6–8 nm). Fluoride adsorption over HIZO is significantly enhanced by its surface modification with β-CD. Predominantly, the pseudo-second order kinetic model for adsorption of fluoride over CHIZO was found to be the best fit. The monolayer adsorption capacity of CHIZO is notably higher than commonly found adsorbents. Thermodynamic modeling envisages that the adsorption process over CHIZO is exothermic in nature. Additionally, phosphate and sulphate show an adverse effect on fluoride adsorption. Fluoride adsorption over CHIZO occurs through the formation of H-bonding with surface –OH groups. Modification of the surface of the metal oxides/hydroxides with β-CD results in enhanced sorption of inorganic pollutants, such as fluorides, and thus CHIZO could be used to efficiently sequester fluoride from water since 0.9 g of CHIZO can reduce the fluoride level to below 1.0 mg L−1 from one-litre of fluoride spiked (5.0 mg L−1) natural water sample.
Acknowledgements
Authors are grateful to the Head, Department of Chemistry, the Vice-Chancellor, Presidency University, Kolkata, India and Sripat Singh College for providing laboratory facilities. One of the authors (I. Saha) is thankful to UGC, New Delhi for minor research project (MRP) grant vide no. PSW-103/14-15, dated 02 Feb, 2015 for carrying out this work.
References
- H. E. Shortt, C. G. Pandit and T. N. Raghavachari, Indian Med. Gaz., 1937, 72, 396–398 CAS.
- Guidelines for drinking-water quality. Volume-1 recommendations, World Health Organization, Geneva Search PubMed.
- WHO, Chemical fact sheets: fluoride, in Guidelines for drinking water quality (electronic resource): incorporation first addendum. Recommendations, Geneva, 3rd edn, 2006, vol. 1, pp. 375–377 Search PubMed.
- UNICEF Statement on Fluoride in Water, 2004, http://www.fluoride.org.uk/statements/unicef.htm Search PubMed.
- S. Jagtap, M. K. Yenkie, N. Labhsetwar and S. Rayalu, Chem. Rev., 2012, 112, 2454–2466 CrossRef CAS PubMed.
- Meenakshi and R. C. Maheshwari, J. Hazard. Mater., 2006, 137(1), 456–463 CrossRef CAS PubMed.
- F. Lou and K. Inoue, Solvent Extr. Ion Exch., 2004, 22, 305–322 CrossRef.
- S. Dey, S. Goswami and U. C. Ghosh, Water, Air, Soil Pollut., 2004, 158, 311–323 CrossRef CAS.
- S. Goswami, S. Dey and U. C. Ghosh, Chem. Environ. Res., 2004, 13, 117–126 Search PubMed.
- K. Biswas, D. Bandhopadhyay and U. C. Ghosh, Adsorption, 2007, 13, 83–94 CrossRef CAS.
- K. Biswas, S. K. Saha and U. C. Ghosh, Ind. Eng. Chem. Res., 2007, 46, 5346–5356 CrossRef CAS.
- K. Biswas, K. Gupta and U. C. Ghosh, Chem. Eng. J., 2009, 149, 196–206 CrossRef CAS.
- I. Saha, K. Gupta, S. Chakraborty, D. Chatterjee and U. C. Ghosh, J. Ind. Eng. Chem., 2014, 20, 1741–1751 CrossRef CAS.
- D. Bonacchia, A. Caneschia, D. Gatteschia, C. Sangregorioa, R. Sessolia and A. Falqui, J. Phys. Chem. Solids, 2004, 65, 719–722 CrossRef.
- A. Z. M. Badruddoza, A. S. H. Tay, P. Y. Tan, K. Hidajat and M. S. Uddin, J. Hazard. Mater., 2011, 185, 1177–1186 CrossRef CAS PubMed.
- A. Z. M. Badruddoza, Z. B. Z. Shawona, T. W. J. Daniela, K. Hidajat and M. S. Uddin, Carbohydr. Polym., 2013, 91, 322–332 CrossRef CAS PubMed.
- S. D. Mhlanga, B. B. Mamba, R. W. Krause and T. J. Malefetse, J. Chem. Technol. Biotechnol., 2007, 82, 382–388 CrossRef CAS.
- E. M. Martin Del Ville, Process Biochem., 2004, 39, 1033–1046 CrossRef.
- E. Norkus, J. Inclusion Phenom. Macrocyclic Chem., 2009, 65, 237–248 CrossRef CAS.
- A. Ponchel, S. Abramson, J. Quartararo, D. Bormann, Y. Barbaux and E. Monflier, Microporous Mesoporous Mater., 2004, 75, 261–272 CrossRef CAS.
- A. Badruddoza, G. S. S. Hazel, K. Hidajat and M. S. Uddin, Colloids Surf., A, 2010, 367, 85–95 CrossRef CAS.
- R. Zhang, L. Li, J. Feng, L. Tong, Q. Wang and B. Tang, ACS Appl. Mater. Interfaces, 2014, 6, 9932–9936 CAS.
- L. Fang, Y. Li, Z. Chen, W. Liu, J. Zhang, S. Xiang, H. Shen, Z. Li and B. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 19951–19957 CAS.
- R. Fuhrer, I. K. Herrmann, E. K. Athanassiou, R. N. Grass and W. J. Stark, Langmuir, 2011, 27(5), 1924–1929 CrossRef CAS PubMed.
- R. Zhao, Y. Wang, X. Li, B. Sun and C. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 26649–26657 CAS.
- A. Alsbaiee, B. J. Smith, L. Xiao, Y. Ling, D. E. Helbling and W. R. Dichte, Nature, 2016, 529, 190–194 CrossRef CAS PubMed.
- L. S. Clesceri, A. E. Greenberg and A. D. Eaton, Standard Methods for the Examination of Water and Wastewater, American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF), Washington, DC, 20th edn, 1998, pp. 4–82 Search PubMed.
- M. B. Babic, M. J. Milonjic and B. V. Kaludierovic, Carbon, 1999, 37, 477–481 CrossRef.
- M. Kosmulski, J. Colloid Interface Sci., 2009, 337, 439–448 CrossRef CAS PubMed.
- A. Ghosh, S. Chakrabarti, K. Biswas and U. C. Ghosh, Appl. Surf. Sci., 2014, 307, 665–676 CrossRef CAS.
- E. Kumar, A. Bhatnagar, M. Ji, W. Jung, S. H. Lee, S. J. Kim, G. Lee, H. Song, J. Y. Choi, J. S. Yang and B. H. Jeon, Water Res., 2009, 43, 490–498 CrossRef CAS PubMed.
- Y. Tang, X. Guan, J. Wang, N. Gao, M. R. McPhail and C. C. Chusuei, J. Hazard. Mater., 2009, 171, 774–779 CrossRef CAS PubMed.
- X. Zhao, J. Wang, F. Wu, T. Wang, Y. Cai, Y. Shi and G. Jiang, J. Hazard. Mater., 2010, 173, 102–109 CrossRef CAS PubMed.
- M. G. Sujana and S. Anand, Desalination, 2011, 267, 222–227 CrossRef CAS.
- Y. S. Ho, Adsorption, 2004, 10, 151–158 CrossRef CAS.
- M. G. Sujana, H. K. Pradhan and S. Anand, J. Hazard. Mater., 2009, 161, 120–125 CrossRef CAS PubMed.
- V. K. Gupta, I. Ali and V. K. Saini, Water Res., 2007, 41, 3307–3316 CrossRef CAS PubMed.
- S. Ghorai and K. K. Pant, Sep. Purif. Technol., 2005, 42, 265–271 CrossRef CAS.
- L. Liu, Z. Cui, Q. Ma, W. Cui and X. Zhang, RSC Adv., 2016, 6, 10783–10791 RSC.
- A. A. M. Daifullah, S. M. Yakout and S. A. Elreefy, J. Hazard. Mater., 2007, 147, 633–643 CrossRef CAS PubMed.
- E. Redente, L. Szente and J. Szejtli, J. Pharm. Sci., 2001, 90, 979–986 CrossRef.
- Y. Domi, K. Ikeura, K. Okamura and K. Shimazu, Langmuir, 2011, 27, 10580–10586 CrossRef CAS PubMed.
- V. Agarwal, G. W. Huber, W. C. Conner Jr and S. M. Auerbach, J. Chem. Phys., 2011, 135, 134506 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16567b |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio) surface is modified in an optimized fashion with β-CD. The molecule has several –OH groups which may anchor metal oxides with the generation of a metal oxide–β-CD composite and this feature can be used to entrap and remove contaminants from aqueous solution through the formation of inclusion complexes with β-CD.23–26
1 mole ratio) surface is modified in an optimized fashion with β-CD. The molecule has several –OH groups which may anchor metal oxides with the generation of a metal oxide–β-CD composite and this feature can be used to entrap and remove contaminants from aqueous solution through the formation of inclusion complexes with β-CD.23–26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of FeCl3 and ZrOCl2 admixture followed by adjustment of the pH to ∼7.0, aging, filtration, drying and sieving as before. The fabricated host–guest complex between the β-CD and HIZO composites was formed using the above procedure. The synthesized materials were optimized for the removal efficiency of fluoride and the maximum efficiency was achieved for the molar composition of β-CD
1 of FeCl3 and ZrOCl2 admixture followed by adjustment of the pH to ∼7.0, aging, filtration, drying and sieving as before. The fabricated host–guest complex between the β-CD and HIZO composites was formed using the above procedure. The synthesized materials were optimized for the removal efficiency of fluoride and the maximum efficiency was achieved for the molar composition of β-CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr as 0.2
Zr as 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Tables 1 and 2).
1 (Tables 1 and 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3
0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr optimization for fluoride adsorption at pH 7.0 from watera
Zr optimization for fluoride adsorption at pH 7.0 from watera
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr
Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc
Kc
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc = ΔS°/2.303R − (ΔH°/2.303R)1/T
Kc = ΔS°/2.303R − (ΔH°/2.303R)1/T







