An aerosol synthesized CeO2:Eu3+/Na+ red nanophosphor with enhanced photoluminescence†
Byeong Ho Mina,
Jong Chan Leea,
Kyeong Youl Jung*a,
Dae Sung Kimb,
Byung-Ki Choic and
Wkang-Jung Kangc
aDepartment of Chemical Engineering, Kongju National University, 1223-4 Cheonan-Daero, Seobuk-gu, Cheonan, Chungnam 331-717, Republic of Korea. E-mail: kyjung@kongju.ac.kr; Fax: +82-41-554-2640; Tel: +82-41-521-9365
bEco-Composite Materials Center, Korea Institute of Ceramic Engineering & Technology (KICET), 101, Soho-ro, Jinju-si, Gyeongsangnam-do, Republic of Korea
cCQV Co., Ltd, 144, Seongjung-ro, Jincheon-eup, Jincheon-gun, Chungcheong Buk-do, Republic of Korea
First published on 22nd August 2016
Abstract
In this work, CeO2:Eu3+/Na+ nanoparticles with high photoluminescence were prepared via a conventional spray pyrolysis process using ethylene glycol (EG) as an organic additive. CeO2:Eu had intensive emission due to the 5D0 → 7F1 (orange) or 5D0 → 7F2 (red) transition of Eu3+. The R/O emission ratio showed a linear increment with the Eu concentration, which was attributed to the reduction in the local symmetry of Eu3+-substituted sites. The optimal Eu concentration to achieve the highest emission intensity was 4 at%, and the luminescence quenching was revealed to occur through a dipole–dipole interaction. The use of EG as a chemical additive could prepare CeO2:Eu nanoparticles of smaller than 80 nm. Also, the emission intensity of nanoparticles was enhanced about 16.5 times higher via codoping Na+ with Eu3+ in the CeO2 matrix, which was attributed to the charge compensation as well as the increase of crystallite size. The use of EG and co-dopant Na+ was proved to be a simple and excellent way to synthesize highly luminescent nanoparticles via spray pyrolysis.
1. Introduction
Rare-earth doped nano-sized phosphors are known as a key material widely used in displays,1–3 lamps,4,5 security inks,6 solar cells7–9 and bio imaging.10–13 In general, phosphors consist of a host and activator. The luminescence characteristics strongly depend on the host composition as well as the type and concentration of activators. To meet the requisition of the application area, a lot of efforts have been made to develop highly efficient phosphors emitting desired properties including color, shape, and size through various synthetic methods. For example, red phosphors can be easily prepared by doping Eu3+ activator into host materials.14–17 The main red emission of Eu3+-doped phosphors is associated with the 5D0 → 7F2 transition of Eu3+. To achieve high emission intensity, host materials should absorb excitation light as much as possible, and an energy transfer to activators should occur effectively. Thus, the choice of host matrix with activator is critical for designing high luminescent phosphor materials.CeO2 has been used in various research areas in optical coating, oxygen conductor, sensor, catalyst and abrasive materials because it has high refractive index, optical transparency, high dielectric constant, and chemical stability.18–22 Also, CeO2 has gained much attention as a host matrix as well as an activator of phosphor materials because it has a strong absorption of UV light through the charge-transfer (CT) transition from O2− to Ce4+ and easily takes part in the energy transfer to other activators.23–27 As a host matrix of phosphor, the emission of CeO2 itself is weak. Thus, a suitable activator doping in CeO2 matrix is needed to achieve good emission characteristics. For example, Sm3+-doped CeO2 shows a strong orange emission due to a 4G5/2 → 6H5/2 transition.28 Eu3+-doped CeO2 is also known to have a sharp orange or red emission due to the 5D0 → 7Fj transition.29,30 Therefore, Eu-doped ceria has been synthesized by various methods including a conventional solid-state reaction, homogenous precipitation, and sol–gel process.
In terms of preparing nanophosphor particles, the single-component oxides such as CeO2, ZnO, Y2O3 and Gd2O3 are more frequently considered because high-phase purity can be achieved at relatively low temperatures. In general, rare-earth doped oxides are needed to be calcined at high temperature in order to achieve high crystalline as well as the activation of activators. In this step, as-prepared nanoparticles are easy to be agglomerated, and frequently it is hard to keep their original nanoparticle size, because a particle growth occurs at high temperature. Therefore, the preparation strategy should be established well to obtain high luminescent phosphor nanoparticles.
Spray pyrolysis as a representative aerosol process have been used to prepare various functional materials because it has many advantages including a simple process, continuous production, easy to control the composition of multi-component oxides, and feasible to prepare fine-sized spherical particles with narrow size distribution from a relatively cheap salt precursors with high solubility in water. Since one particle comes from one droplet in the spray pyrolysis, all ingredients can be mixed, homogeneously. As a result, when the spray pyrolysis is applied to synthesize phosphors, the activator ions can be distributed uniformly within the whole of host matrix. Therefore, the spray pyrolysis process has been used for the synthesis of high luminescent phosphor materials.31–36 The particle size in the spray pyrolysis can be controlled by the concentration (C) of precursor solution because the particle size is proportional to C1/3. That is, the size reduction of particles is achieved easily by lowering the precursor concentration. The production quantity, however, is dramatically reduced by lowering the concentration in order to prepare nanoparticles of less than 100 nm. Given this, the approach lowering precursor concentration to obtain nano-sized particles is thought to be not a good method in terms of the practical application of the spray pyrolysis. Thus, the development of a new synthetic strategy profitable for the mass preparation of nanoparticles is a challenging work in the spray pyrolysis. Recently, we have developed a simple and effective way that is to change the mechanism of particle formation via adding some additives in the spray solution.17 The key concept is to produce very hollow particles with a thin shell layer. These hollow particles can be disintegrated into nanoparticles via a recrystallization process during the calcination at high temperatures. In this work, Eu-doped CeO2 nanoparticles with high luminescence were synthesized by the spray pyrolysis. The luminescence properties including the luminescence quenching mechanism of CeO2:Eu3+ were investigated by changing the concentration of Eu activator. Ethylene glycol as an organic additive was introduced into the precursor solution with intention of preparing nano-sized CeO2:Eu3+ particles via the spray pyrolysis. Finally, Na+ ions were codoped to improve the emission intensity of CeO2:Eu3+ nanoparticles.
2. Experimental
2.1 Materials and sample preparation
Cerium nitrate (Aldrich, 99%), europium oxide (Aldrich, 99.99%), ethylene glycol (Aldrich) and sodium bicarbonate (NaHCO3, Aldrich) were used as the starting materials. A conventional ultrasonic spray pyrolysis was used to prepare CeO2:Eu/Na particles. The spray pyrolysis (Fig. S1†) consists of an aerosol generator with 6 vibrators of 1.7 MHz, quartz tube (length = 1200 mm and ID = 55 mm), and a Teflon bag filter. The precursor spray solution was prepared by the following procedure. First, Eu2O3 was dissolved as a nitrate form by using nitric acid. Thereafter, the cerium nitrate was added to the Eu activator solution, and the total solution was adjusted to be 500 mL by adding purified water. The total concentration of precursor solution was fixed at 0.2 M. The Eu contents (x) in Ce1–xO2:Eux were varied from 0.02 to 0.15. To prepare nano-sized CeO2:Eu particles, EG of 0.06 to 0.20 M was additionally dissolved into the precursor solution. NaHCO3 was also dissolved with EG into the precursor solution. The content of Na+ ion was fixed at the same concentration with the Eu concentration.The prepared precursor solution was atomized to produce droplets which were carried by air of 40 liter per min into the quartz reactor of 900 °C. The produced powders were collected by the bag filter installed at the end of the quartz reactor. The resulting CeO2:Eu3+ or CeO2:Eu3+/Na+ powders were calcined at the temperature range from 900 °C to 1100 °C for 3 h.
2.2 Characterization
The excitation and emission properties of the prepared phosphors were measured by a spectrophotometer (PerkinElmer, LS 50). The microstructure was observed by high resolution scanning electron microscopy (HR-SEM, Hitachi S4800) and field-emission transmission electron microscopy (FE-TEM, JEOL-2100F) at the Korea Basic Science Institute (KBSI, Suncheon). The crystallographic information of the prepared powders was identified by an X-ray diffraction measurement (XRD, Rigaku, MiniFlex600).3. Results and discussion
3.1 Effect of Eu concentration on emission properties
Fig. 1 shows the photoluminescence (PL) and excitation spectrum (PLE) of CeO2:Eu0.02 phosphor calcined at 1000 °C. The emission peaks observed are corresponding to the 5D0 → 7Fj (j = 1, 2, 3, 4) transition of Eu3+. The dominant peak is located at 592 nm due to the 5D0 → 7F1 transition. The PLE spectrum shows three peaks around 272 nm, 368 nm and 470 nm. The excitation peak around 470 nm is due to the f–f transition of Eu3+ ions. In CeO2:Eu3+, the charge transfer transition takes place from host to Eu3+. The light absorption occurs initially via the charge transfer (CT) from O2− to Ce4+. Thereafter, the absorbed light energy is transferred from Ce4+–O2− to Eu3+.29 The excitation peak observed in the wavelength region of 300–400 nm is ascribed to the charge transfer from O2− to Ce4+. There is a small excitation peak in the wavelength range of 250–300 nm, which is not due to the Ce–O charge transfer. In Eu3+-doped oxides, the direct O2−–Eu3+ charge transfer can easily occur. The energy for the O2−–Eu3+ charge transfer is different depending on the host materials. According to literatures,37,38 the O2−–Eu3+ charge transfer takes place at the energy level higher than about 3.87 eV (320 nm). Thus, the small peak observed in the wavelength range of 250–300 nm is thought to be originated from the charge transfer directly from O2− to Eu3+. | ||
| Fig. 1 Excitation (a) and emission (b) spectrum of CeO2:Eu3+ prepared by spray pyrolysis at the Eu content of 2.0 at% and the calcination temperature of 1000 °C. | ||
The emission spectra of CeO2:Eu3+ particles were monitored by changing the activator concentration. Fig. 2(a) shows the emission spectra obtained with the Eu content (x) from 0.02 to 0.15. The Eu3+ concentration affects both the emission intensity and the dominant wavelength. The emission peak intensities at 592 nm, 612 nm and 632 nm were displayed in Fig. 2(b) as a function of the Eu3+ content, x in Ce1–xO2:Eux. The highest intensity was observed at x = 0.04, which is same for three emission peaks observed. When the Eu content (x) is 0.10 or larger, the dominant emission peak shifts from 592 nm (5D0 → 7F1) to 612 nm (5D0 → 7F2). The dominant emission peaks of Eu3+-doped oxides are known to depend on the symmetry of crystal sites. CeO2 has a cubic fluorite-type structure in which the Ce4+ ions occupy highly symmetric sites (inversion center). The magnetic dipole transition 5D0 → 7F1 becomes a dominant emission path when Eu3+ ions are located in a site with an inversion symmetry in which electrical dipole transition is forbidden, and the transition is insensitive to the crystal field environment.29 In a site without inversion symmetry, the electrical dipole transition is allowed and the 5D0 → 7F2 transition becomes a dominant peak. Also, the 5D0 → 7F2 transition of Eu3+ is known to be hypersensitive to the crystal field environment. Therefore, the change in the position of dominant emission peaks reflects the change in the symmetry of Eu3+-substituted sites. In nanocrystalline structure, Eu3+ can be located near the grain surface as well as in the inside of the grain. The Eu3+-substituted sites in the center of grain have a relatively higher symmetry compared with that near the grain surface. Thus, the spectral peak due to the 5D0 → 7F2 transition is observed as the separated two peaks (612 nm and 632 nm). In CeO2:Eu, Eu3+ ions are incorporated into Ce4+ sites. To balance the charge difference between two cations, oxygen vacancies are formed by the following reaction.27
| (1 − x)CeO2 + 0.5xEu2O3 → xEuCe + 0.5xVO + (1 − x)CeCe + (2 − 0.5x)OO | (1) |
 | ||
| Fig. 2 The effect of Eu3+ concentration on (a) emission spectra, (b) emission intensity, (c) ratio of emission intensity, and (d) color coordinates of CeO2:Eu. | ||
Fig. 3 shows the XRD patterns of CeO2:Eu powders prepared by spray pyrolysis. The resulting XRD patterns are well indexed as a pure cubic CeO2 phase (JCPDS # 65-2975), and no impurity peaks are observed regardless of the Eu3+ concentration. The main XRD peak positions shift to a lower diffraction angle with increasing the Eu3+ concentration up to 5% (x = 0.05). This peak shift is because the radius of Eu3+ (0.113 nm) is larger than that of Ce4+ (0.102 nm). As a result, the Eu3+ substation into the Ce4+ sites inflates the unit cell volume of CeO2. The lattice constant are calculated using the relation of d2 = a2/(h2 + k2 + l2) and d = 0.5λ/sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, using the peak information for the (111) plane. The resulting lattice constants are shown in Fig. S2.† The lattice constant increases monotonically with the increase of Eu3+ content up to x = 0.05. At the Eu3+ contents over x = 0.05, there is no significant variation in the lattice constant. The crystallinity of oxides can be evaluated from the crystallite size. In general, the larger crystallite size reflects the higher crystallinity. The increase of crystallinity means the reduction of defect sites where the photo-excited electrons are consumed without luminescence. Thus, the generation of oxygen vacancies affects the crystallinity. The crystallite sizes of CeO2:Eu3+ particles, which is calculated from the Scherer formula and shown in Fig. S2,† tend to slightly decrease with increasing the Eu3+ content. Given this, the increase of the Eu3+ content generate more oxygen vacancies, which is attributed to reduce the symmetry of Eu3+-substituted sites. As a result, the increase of Eu3+ concentration makes it possible to accelerate the 5D0 → 7F2 transition.
θ, using the peak information for the (111) plane. The resulting lattice constants are shown in Fig. S2.† The lattice constant increases monotonically with the increase of Eu3+ content up to x = 0.05. At the Eu3+ contents over x = 0.05, there is no significant variation in the lattice constant. The crystallinity of oxides can be evaluated from the crystallite size. In general, the larger crystallite size reflects the higher crystallinity. The increase of crystallinity means the reduction of defect sites where the photo-excited electrons are consumed without luminescence. Thus, the generation of oxygen vacancies affects the crystallinity. The crystallite sizes of CeO2:Eu3+ particles, which is calculated from the Scherer formula and shown in Fig. S2,† tend to slightly decrease with increasing the Eu3+ content. Given this, the increase of the Eu3+ content generate more oxygen vacancies, which is attributed to reduce the symmetry of Eu3+-substituted sites. As a result, the increase of Eu3+ concentration makes it possible to accelerate the 5D0 → 7F2 transition.
Several properties such as lattice constant, crystallite size, Eu content and R/O ratio were summarized in Table 1 including the previous works.29,39 In polycrystalline CeO2:Eu, Eu3+ ions can be placed in the inside of the grain or near the grain surface. Eu3+ ions substituted in the inside lattice of the grain dominantly contribute to the expansion of lattice volume of CeO2. On the contrary, the Eu3+ ions located in the sites near the grain boundary of polycrystalline CeO2 particles do not much contribute to the expansion of lattice volume. If all Eu3+ ions are substituted into the Ce4+ sites in the inside of the grain of CeO2, the lattice constant should increase monotonically with increasing the concentration of Eu3+ ions. In Fig. S3,† the lattice constants and the R/O ratio of CeO2:Eu particles prepared in this work were compared with the values reported in previous literatures.29,40,41 The overall dependence of lattice constant on the concentration of Eu3+ ions is in good agreement with the expectation although it does not have linearity. As shown in Fig. S3(b),† the R/O ratio increases linearly as increasing the Eu3+ concentration regardless of the preparation method. But, the slope of the R/O ratio with respect to the Eu3+ content is different by the preparation method. This result reflects that the symmetry of the Eu3+-substituted sites is affected by the preparation method even if the Eu3+ concentration is same. In special, the R/O ratio of CeO2:Eu phosphor prepared by a solid-state reaction (SSR) are much larger compared with other two methods (sol–gel and spray pyrolysis). That is, CeO2:Eu3+ prepared by the spray pyrolysis (SP) has more Eu3+-substituted sites having the inversion symmetry than the solid-state reaction. As shown in Table 1, CeO2:Eu prepared by spray pyrolysis has larger lattice constants than the sol–gel synthesized CeO2:Eu at the same Eu3+ concentration. This difference could be connected with the crystallite size. The crystallite size of CeO2:Eu prepared by the spray pyrolysis is much larger than that prepared by the sol–gel reaction, which is due to the difference in calcination temperature. The smaller in the crystallite size, the larger in the grain surface. Thus, Eu3+ ions could exist more in the inside than near the surface of the grain as the crystallite size increases. Therefore, the lattice volume expansion by substituting Eu3+ ions into the CeO2 matrix could be enlarged as the crystallite size increases even if the Eu3+ concentration is same.
| Preparation method | Temp. [°C] | Dc [nm] | a [Å] | Eu3+ [%] | R/O |
|---|---|---|---|---|---|
| a The values were calculated from the data in the reference. | |||||
| Sol–gel (ref. 29) | 600 | 15.2 | 5.385 | 1 | 0.081 |
| 700 | 27.8 | 5.392 | 1 | 0.131 | |
| 800 | 52.8 | 5.401 | 1 | 0.222 | |
| 700 | 27.5 | 5.398 | 5 | 0.667 | |
| 23.6 | 5.401 | 10 | 1.351 | ||
| Solid-state reaction (ref. 39) | 1360 | — | 5.416 | 1 | 0.625a |
| — | — | 5 | 1.728a | ||
| — | 5.423 | 10 | 2.749a | ||
| Spray pyrolysis (this work) | 1000 | 43.9 | 5.391 | 2 | 0.519 |
| 43.7 | 5.427 | 5 | 0.678 | ||
| 43.4 | 5.428 | 10 | 1.008 | ||
To investigate the luminescence quenching behaviour, the total emission intensity was calculated by integrating the emission spectra in the wavelength range from 550 nm to 750 nm. The resulting intensities are shown in Fig. 4 as a function of Eu3+ content. Basically, the luminescence quenching is due to the non-radiative energy transfer of excitation energy. If activators are placed within a critical distance, the non-radiative energy transfer between activators can occur via an exchange interaction or an electric multipolar interaction. The exchange interaction could be possible when the critical distance (Rc) is less than about 5 Å. According to Blasse and Bril,42 the critical distance is expressed by the following equation:
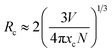 | (2) |
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K − ln
K − ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β). From the slope in the linear plot of ln(I/x) vs. ln(x), which is shown in the inset of Fig. 4, the Q value can be obtained. The obtained slope is about – 1.72, which means that Q is closed to 6. Therefore, the luminescence quenching was concluded to occur through the dipole–dipole interaction.
β). From the slope in the linear plot of ln(I/x) vs. ln(x), which is shown in the inset of Fig. 4, the Q value can be obtained. The obtained slope is about – 1.72, which means that Q is closed to 6. Therefore, the luminescence quenching was concluded to occur through the dipole–dipole interaction.
 | ||
| Fig. 4 Integrated emission intensity as a function of the Eu3+ content and the inset is a plot of ln(I/x) vs. ln(x). | ||
3.2 EG effect on particle size and luminescence intensity
When oxide particles are prepared by spray pyrolysis using the nitrate precursor solution, they usually have a spherical shape with a hollow structure because the precipitation of salts during a drying step begins at the surface of droplets. The particle morphology can be controlled by changing the characteristics in the drying and precipitation steps in the spray pyrolysis process. In this work, ethylene glycol (EG) was used as an organic additive with the intention of preparing CeO2:Eu3+ nanoparticles via controlling the particle formation mechanism in the spray pyrolysis. Fig. 5 shows SEM photos of CeO2:Eu3+ particles prepared with and without EG. The CeO2:Eu3+ particles prepared without EG have spherical morphology and hollow structure. The shell thickness is thick, which makes it possible to maintain the spherical shape after the calcination at 1000 °C. As shown in the SEM photo focused on the surface of particles prepared without EG (Fig. 5(c)), the primary particles have the size of 150–200 nm and exist as a hard aggregate. Whereas, for the case of using EG, the as-prepared particles (Fig. 5(d)) have a very hollow structure with a thin shell layer, and the particle size is larger compared with the particles prepared without EG. | ||
| Fig. 5 SEM photos of CeO2:Eu3+ particles prepared from the precursor solution without (a–c) and with (d–f) EG. | ||
A particle formation mechanism is schematically displayed in Scheme 1. During the evaporation of water going through the hot quartz tube, EG is not vaporized because its boiling point is higher than water. Instead, EG coexists with the precipitated salt precursors in the surface shell layer or inside droplets. The formed shell layer becomes sticky and flexible due to the concentrated EG. Then, the shell layer can be inflated like a balloon due to the increase of the inner pressure by the temperature increase as well as a lot of gaseous compounds generated from the pyrolysis of precursors. As a result, very hollow CeO2:Eu3+ particles with a thin layer could be prepared by adding EG to the precursor solution. Since the residence time of droplets in the reactor is short, which is less than about 3 s, the as-prepared hollow particles consist of the pyrolyzed cerium oxide and some carbon residues originated from the incomplete pyrolysis of EG. During the post thermal treatment which is needed for the crystallization of CeO2 and the activation of Eu3+, nano-sized crystals are formed in the thin shell layer. The carbon residues make it possible to suppress the excess crystal growth as well as disintegrating the formed nano-sized primary particles. Fig. 5(e) and (f) are SEM images for the CeO2:Eu3+ particles obtained after the calcination of the precursor powder prepared from the spray solution containing EG. Some of particles show a fractured structure. The primary particles have the size of less than 100 nm, and they are necked weakly. Fig. 6 shows the TEM image of a hollow CeO2:Eu3+ particle prepared by using EG and the elementary mapping for Ce and Eu components. The TEM images indicates that the spherical particle have a very hollow structure having a thin shell of several tens nanometers. The element mapping images of the hollow CeO2:Eu3+ particle indicates the uniform distribution of the Eu component over the entire hollow sphere. Given this, Eu activators are uniformly substituted into CeO2 matrix. According to the TEM image of the fractured particles (Fig. 6(d)), CeO2:Eu3+ particles prepared form the EG-added precursor solution have the size of smaller than 80 nm.
 | ||
| Fig. 6 TEM image (a and d) and element mapping (b and c) of CeO2:Eu3+ hollow particles prepared from the EG-containing precursor solution. | ||
Fig. 7 shows the excitation (PLE) and emission (PL) spectra of CeO2:Eu3+ particles prepared from the precursor solution with and without EG. The overall shapes of the PL and PLE spectra do not change, significantly. The emission intensity ratio of 5D0 → 7F2 (612 nm) to 5D0 → 7F1 (592 nm) are 0.712 and 0.778 for the CeO2:Eu3+ particles with and without EG, respectively. This indicates that the symmetry degree of the Eu3+-substituted sites is not significantly changed. The emission intensity, however, is largely reduced by adding EG to the precursor solution. The emission intensity is generally known to be lowered with reducing the particle size because a lot of surface defects are generated. As shown in Fig. 5(f) and 6(d), the primary particle size is largely reduced by the use of EG. Therefore, for the sample prepared by using EG, the reason for the large reduction of the emission intensity is because of the reduction in the primary particles size. Fig. S4† shows the effect of EG concentration on the PL intensity of CeO2:Eu3+. The emission intensity are gradually reduced by increasing the EG concentration up to 0.1 M. When the EG concentration is over 0.1 M, however, CeO2:Eu3+ nanoparticles have no significant changes in the emission intensity. For the CeO2:Eu3+ particles prepared at the EG concentration of 0.1 M, the calcination temperature was changed from 900 °C to 1100 °C. The morphology and the emission intensity were monitored with the temperatures (Fig. S5 and S6†). The primary particle size was slightly increased with increasing the calcination temperature. However, there is no significant agglomeration between the primary particles regardless of the calcination temperature. The emission intensity is monotonically increased with increasing the temperature due to the improvement of crystallinity. The results indicates that the calcination temperature of 1100 °C is no problem in terms of preparing good luminescent nano-sized CeO2:Eu particles by the spray pyrolysis.
 | ||
| Fig. 7 Comparison in the excitation and emission spectra of CeO2:Eu3+ (4.0 at%) particles prepared from the precursor solution with and without EG. | ||
3.3 Na+ codoping effect on luminescence intensity and particle size
CeO2 matrix is known to have some percentage of Ce3+ sites in the grain boundary. Also, oxygen vacancies are generated by substituting Eu3+ into Ce4+ in a cubic unit cell.44 Then, the photo-excited holes and electrons can be consumed by Ce3+ and oxygen vacancy, respectively. The presence of such defects is not good for the radiative recombination, resulting in the decrease of luminescence intensity. Therefore, the emission intensity of phosphor is generally improved via a calcination process at high temperature due to the reduction of defects sites. In CeO2:Eu3+, the charge of host cation (Ce4+) is different with the charge of activator (Eu3+). The oxygen vacancies can be compensated by codoping monovalent metal ions. In this work, Na+ ions were codoped with Eu3+ in order to compensate the unbalanced charge between Ce4+ and Eu3+. At the optimal Eu content, which was 4 at% with respect to Ce in terms of the emission intensity, NaHCO3 as the Na+ precursor was added to the precursor solution containing EG (0.1 M). The content of NaHCO3 was equal to the atomic percentage of Eu3+.Fig. 8(a) and (b) show the excitation and emission spectra of CeO2:Eu3+/Na+ nanoparticles. The excitation peak for the Ce–O charge transfer is substantially improved. The emission intensity of CeO2:Eu3+/Na+ is enhanced about 16.5 times of CeO2:Eu3+. This result indicates that the charge compensation via the Na+ codoping effectively occurs with removing the oxygen vacancies. As a result, the Na+ codoping makes it possible to prohibit the non-radiative recombination of photo-excited electrons. Fig. 8(c) shows the XRD patterns of CeO2:Eu3+/Na+ with CeO2:Eu3+. The Na+ codoping do not generate any impurity peaks. The crystallite size of CeO2:Eu3+/Na+ is about 61 nm, which is much larger than that (about 44 nm) of CeO2:Eu3+. Given this, the crystallinity of CeO2 is largely improved by the Na+ codoping. The main peak of XRD slightly shifts to the large diffraction angle, indicating that Na+ ions are well inserted in the oxygen vacancy. For the CeO2:Eu3+/Na+ sample, the PL intensity were monitored by changing the calcination temperature from 800 °C to 1100 °C. The results are shown in Fig. S7.† No significant change in the emission intensity was observed except for 800 °C.
 | ||
| Fig. 8 Effect of Na+ cooping: (a) excitation, (b) emission (inset – emission photo under the excitation of 365 nm hand UV) and (c) XRD patterns of CeO2:Eu3+ and CeO2:Eu3+/Na+ nanoparticles. | ||
Fig. 9 shows SEM, TEM and element mapping of CeO2:Eu3+/Na+ calcined at 1000 °C. Compared with the case that no NaHCO3 is used, the particle size is enlarged. This is because the NaHCO3 acts as a flux which is known to accelerate the crystal growth. According to the TEM image, the primary particles have the size of 150–200 nm with an agglomeration-free structure. The selected area electron diffraction (SAED) pattern (the inset of Fig. 9(b)) indicates the high crystalline nature of the CeO2:Eu3+/Na+ particles, which is in good agreement with the XRD data in Fig. 8(c). It is notable that there is no significant agglomeration between the prepared CeO2:Eu3+/Na+ primary even though the size is increased. The element mapping data reveals that Na+ and Eu3+ ions are well distributed in host matrix. From the results so far achieved, it was elucidated that the Na+ codoping is helpful for improving the emission intensity of Eu-doped ceria because it can successfully compensate the unbalanced charge between Ce4+ and Eu3+ simultaneously with improving the crystallinity.
 | ||
| Fig. 9 (a) SEM and (b) TEM (inset – SAED pattern), and (c) element mapping of CeO2:Eu3+/Na+ nanoparticles. | ||
4. Conclusions
CeO2:Eu3+ and CeO2:Eu3+/Na+ nanoparticles were successfully prepared via a spray pyrolysis process. Luminescence properties, morphology and particle size were systematically studied with varying the Eu3+ concentration, introducing ethylene glycol, and codoping monovalent Na+ ion. A strong excitation observed around 365 nm is attributed to the charge transfer from O2− to Ce4+. In CeO2:Eu3+, Eu3+ ions are mainly located in sites with inversion symmetry. As a result, the strong emission peak at 593 nm due to the 5D0 → 7F1 transition of Eu3+ was observed in a whole range of Eu3+ concentration. The local symmetry of Eu3+, however, was lowered with increasing the Eu3+ concentration due to the lattice distortion as well as the formation of oxygen vacancies, which led to increase the peak at 612 nm due to the 5D0 → 7F2 transition of Eu3+. When the Eu3+ content was over 4%, the concentration quenching of the emission intensity was identified to occur through the dipole–dipole interaction. Introducing EG to the spray solution was found to an effective way to prepare CeO2:Eu nanoparticles of smaller than 80 nm, but the emission intensity was largely lowered due to the reduction of particle size. This lowered emission intensity of CeO2:Eu3+ nanoparticles prepared using EG could be enhanced about 16.5 times higher via codoping Na+ with Eu3+ in CeO2 matrix, which was attributed to the charge compensation as well as the increase of crystallite size. According to the dot mapping of elements, the Ce, Eu, Na and O components were confirmed to have a uniform distribution throughout the nanoparticles without any phase separation. The use of EG and co-dopant Na+ was experimentally identified to be a good way to synthesize high luminescent CeO2:Eu+/Na+ nanoparticles of less than 200 nm via the spray pyrolysis.Acknowledgements
This work was supported by the Technology Innovation Program (Advanced Technology Center, ATC) funded by the Ministry of Trade, industry & Energy, Republic of Korea (Grant no. 10052088).Notes and references
- J. H. Park, Y. S. Jeong, N. W. Jang and J. S. Kim, J. Lumin., 2010, 130, 1415 CrossRef CAS.
- W.-S. Song, K.-H. Lee, Y. R. Do and H. Yang, Adv. Funct. Mater., 2012, 22, 1885 CrossRef CAS.
- S. Thakur and A. K. Gathania, J. Electron. Mater., 2015, 44, 3444 CrossRef CAS.
- H. Menkara, R. A. Gilstrap, T. Morris, M. Minkara, B. K. Wagner and C. J. Summers, Opt. Express, 2011, 19, A972 CrossRef CAS PubMed.
- A. Aboulaich, M. Michalska, R. Schneider, A. Potdevin, J. Deschamps, R. Daloncle, G. Chadeyron and R. Mahiou, ACS Appl. Mater. Interfaces, 2014, 6, 252 CAS.
- B. K. Gupta, D. Haranath, S. Saini, V. N. Singh and V. Shanker, Nanotechnology, 2010, 21, 055607 CrossRef PubMed.
- N. Chander, A. F. Khan, V. K. Komarala, S. Chawla and V. Dutta, Prog. Photovolt: Res. Appl., 2016, 24, 692 CrossRef CAS.
- C. Liu, X. Zhang, J. Li, Y. He, Z. Li, H. Li, W. Guo and W. Xie, Synth. Met., 2015, 204, 65 CrossRef CAS.
- N. Chander, A. F. Khan and V. K. Komarala, RSC Adv., 2015, 5, 66057 RSC.
- A. Sendlmeier and H. H. Gorris, Chem. Soc. Rev., 2015, 44, 1526 RSC.
- B. K. Gupta, V. Rathee, T. N. Narayanan, P. Thanikaivelan, A. Saha, G. S. P. Singh, V. Shanker, A. A. Marti and P. M. Ajayan, Small, 2011, 7, 1767 CrossRef CAS PubMed.
- L. Huo, J. Zhou, R. Wu, J. Ren, S. Zhang, J. Zhang and S. Xu, Opt. Mater. Express, 2016, 6, 1056 CrossRef.
- Y. Xie, W. He, F. Li, T. S. H. Perera, L. Gan, Y. Han, X. Wang, S. Li and H. Dai, ACS Appl. Mater. Interfaces, 2016, 8, 10212 CAS.
- X. Zeng, J. Yuan, Z. Wang and L. Zhang, Adv. Mater., 2007, 19, 4510 CrossRef CAS.
- M. E. Foley, R. W. Meulenberg, J. R. McBride and G. F. Strouse, Chem. Mater., 2015, 27, 8362 CrossRef CAS.
- J. S. Cho, J. Y. Jung and Y. C. Kang, Phys. Chem. Chem. Phys., 2015, 17, 1325 RSC.
- J. S. Cho, K. Y. Jung, M. Y. Son and Y. C. Kang, RSC Adv., 2014, 4, 62965 RSC.
- L. Martinu and D. Poitras, J. Vac. Sci. Technol., A, 2000, 18, 2619 CAS.
- M. J. D. Rushton and A. Chroneos, Sci. Rep., 2014, 4, 6068 CrossRef CAS PubMed.
- L. Wang, H. Huang, S. Xiao, D. Cai, Y. Liu, B. Liu, D. Wang, C. Wang, H. Li, Y. Wang, Q. Li and T. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 14131 CAS.
- J.-X. Feng, S.-H. Ye, H. Xu, Y.-X. Tong and G.-R. Li, Adv. Mater., 2016, 28, 4698 CrossRef CAS PubMed.
- P. Suphantharida and K. Osseo-Asare, J. Electrochem. Soc., 2004, 151, G658 CrossRef CAS.
- C. Sorbello, B. C. Barja and M. Jobbágy, J. Mater. Chem. C, 2014, 2, 1010 RSC.
- Y. Sohn, J. Am. Ceram. Soc., 2013, 96, 3747 CrossRef CAS.
- D. Fang, M. Zhang, Z. Luo, T. Cao, Q. Wang, Z. Zhou, M. Jiang and C. Xiong, Opt. Mater., 2014, 38, 1 CrossRef CAS.
- J. Malleshappa, H. Nagabhushana, B. Daruka Prasad, S. C. Sharma, Y. S. Vidya and K. S. Anantharaju, Optik, 2016, 127, 855 CrossRef CAS.
- S. Shi, M. Hossu, R. Hall and W. Chen, J. Mater. Chem., 2012, 22, 23461 RSC.
- M. Oikawa and S. Fujihara, J. Eur. Ceram. Soc., 2005, 25, 2921 CrossRef CAS.
- L. Li, H. K. Yang, B. K. Moon, Z. Fu, C. Guo, J. H. Jeong, S. S. Yi, K. Jang and H. S. Lee, J. Phys. Chem. C, 2009, 113, 610 CAS.
- X. Liu, S. Chen and X. Wang, J. Lumin., 2007, 127, 650 CrossRef CAS.
- J. S. Cho, S. M. Lee, K. Y. Jung and Y. C. Kang, RSC Adv., 2014, 4, 43606 RSC.
- J. S. Cho, K. Y. Jung and Y. C. Kang, RSC Adv., 2015, 5, 8345 RSC.
- J. H. Kim and K. Y. Jung, J. Lumin., 2011, 131, 1487 CrossRef CAS.
- K. Y. Jung and H. W. Lee, J. Lumin., 2007, 126, 469 CrossRef CAS.
- G. S. Freiria, E. J. Nassar, M. Verelst and L. A. Rocha, J. Lumin., 2016, 169, 844 CrossRef CAS.
- S.-H. Lee, J. I. Choi, Y. J. Kim, J. K. Han, J. Ha, E. Novitskaya, J. B. Talbot and J. McKittrick, Mater. Charact., 2015, 103, 162 CrossRef CAS.
- L. Li and S. Zhang, J. Phys. Chem. B, 2006, 110, 21438 CrossRef CAS PubMed.
- G. Blasse and G. J. Dirksen, J. Electrochem. Soc., 1989, 136, 1550 CrossRef CAS.
- X. Liu, S. Chen, X. Wang and X. Wang, J. Lumin., 2007, 127, 650 CrossRef CAS.
- J. Wu, S. Shi, X. Wang, H. Song, M. Luo and W. Chen, J. Lumin., 2014, 152, 142 CrossRef CAS.
- J. Malleshappa, H. Nagabhushana, S. C. Prashantha, S. C. Sharma, N. Dhananjaya, C. Shivakumara and B. M. Nagabhushana, J. Alloys Compd., 2014, 612, 425 CrossRef CAS.
- G. Blasse and A. Bril, Appl. Phys. Lett., 1967, 11, 53 CrossRef CAS.
- K. Y. Jung and H.-K. Jung, J. Lumin., 2010, 130, 1970 CrossRef CAS.
- D. Avram, M. Sanchez-Dominguez, B. Cojocaru, M. Florea, V. Parvulescu and C. Tiseanu, J. Phys. Chem. C, 2015, 119, 16303 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16551f |
| This journal is © The Royal Society of Chemistry 2016 |


