A series of tetraphenylethene-based benzimidazoles: syntheses, structures, aggregation-induced emission and reversible mechanochromism†
Abstract
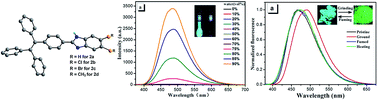
Four tetraphenylethene-based benzimidazoles 5,6-R-2-(4-tetraphenyletheneyl)-1H-benzo[d]imidazoles (R = H (2a), Cl (2b), Br (2c) and CH3 (2d)) were conveniently synthesized in high yields by the cyclization reaction of 4-tetraphenylenthenealdehyde with phenylenediamines. These compounds were structurally characterized and their properties were analyzed by spectroscopy, electrochemistry, X-ray diffraction, thermal stability and theoretical studies. Single crystal X-ray diffraction analyses indicate that their molecular structures in the aggregated state are highly twisted conformations and there exist obvious cavities and/or interface gaps in their crystal packing structures. These four compounds exhibit typical aggregation-induced emission (AIE) properties with relatively high absolute fluorescence quantum yields (30.6–67.5%). These compounds also exhibit reversible mechanochromism behaviors with emission changes between light blue and yellow-green, which are due to the reversible morphological transformation between unconsolidated crystalline state and amorphous state. They are thermally stable with decomposition temperatures above 340 °C.

 Please wait while we load your content...
Please wait while we load your content...