Effect of high-pressure homogenization on structural, thermal and rheological properties of bambara starch complexed with different fatty acids
Abstract
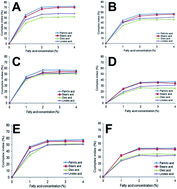
The effect of high-pressure homogenization (HPH) on the degree of complexation of different fatty acids with bambara starch was studied. HPH significantly increased the complexation of bambara starch with palmitic, stearic, oleic and linoleic acids. However, saturated fatty acids generally showed higher complexing ability than unsaturated ones. For all fatty acids, bambara starch showed a higher complex index than corn and potato starches, which could be associated with the variation in amylose contents (22.5–31.5%). The formation of V-amylose crystalline materials was confirmed by XRD with peaks at 2θ = 7.4, 12.9 and 19.9°. Bambara starch–fatty acid complexes displayed significantly higher melting temperatures (95.74–103.82 °C) compared to native uncomplexed starch (77.32 °C). Homogenized bambara starch complexes were non-gelling while unhomogenized complexes produced weak gels, with G′ > G′′ in the range of 0.1–10 Hz. Complexation of bambara starch with fatty acids using HPH may be employed in the production of modified starch with non-gelling properties and higher thermal stability suitable for certain industrial applications.

 Please wait while we load your content...
Please wait while we load your content...