Asymmetric hybrid capacitors based on novel bearded carbon fiber cloth–pinhole polyaniline electrodes with excellent energy density†
Jun Maab,
Shaochun Tanga,
Junaid Ali Syeda and
Xiangkang Meng*a
aInstitute of Materials Engineering, National Laboratory of Solid State Microstructures, College of Engineering and Applied Sciences, Nanjing University, Jiangsu, P. R. China. E-mail: mengxk@nju.edu.cn; Fax: +86 25 83595535; Tel: +86 25 83685585
bElectrical and Mechanical Department, Nantong Science and Technology College, Jiangsu, P. R. China
First published on 17th August 2016
Abstract
It is well known that electronic conductivity and kinetics of ion diffusion determine the large current discharge capacity of electrode materials. Hence, we envisioned construction of an integrated smart structure for which fast ion and electron transfer were guaranteed. This paper presents a novel bearded carbon fiber cloth (CFC) decorated with pinhole polyaniline (PANI) formed by electro-polymerization to achieve excellent electrochemical properties. The pinhole nanostructure of redox-active PANI exposed a high electrolytic-attainable surface area and the bearded CFC would serve as an excellent 3D conductive skeleton, which would supply a direct channel for electron transport. As the current density increased from 1.3 to 16.5 A g−1, the PANI/CFC still maintained a higher specific capacitance; the specific capacitance decreased from 808 F g−1 to 749 F g−1, only ∼7% loss of capacitance, which implies that the PANI/CFC electrodes have excellent rate capability. The optimal composite electrodes achieved a high area-normalized capacitance of 3.3 F cm−2. The asymmetric hybrid capacitors based on optimal composite electrodes were tested in a cell voltage region of 0–1.6 V and exhibited high energy densities with values of 36.35 Wh kg−1 and power densities of 422.15 W kg−1. The results showed that the combination of porous PANI with bearded CFC significantly increased the capacitance of the electrode and enhanced its electrochemical performance.
1. Introduction
Nowadays, growing concerns about environmental pollution with an increasing demand for energy has attracted researchers to develop electrochemical energy-storage devices. Electrochemical capacitors (ECs) have a long cycle life, high power performance and low cost.1–3 However, ECs usually have lower energy densities than that of rechargeable batteries. Energy density can be improved by maximizing the specific capacitance and/or the cell voltage. Asymmetric capacitors can combine battery-type faradic electrodes (as the power source) in order to increase the operation voltage and the energy density.4–6The increasing demand of portable electronics has boosted the development of energy-storage systems that are flexible, lightweight, bendable, ultrathin, and even wearable.7–10 PANI was considered to be the best candidate among conducting polymers due to its ease of synthesis with good environmental stability, cost-effectiveness and redox reversibility.11 Numerous methods have been reported for the synthesis of PANI and its application as a supercapacitor.12,13 Horng et al. reported PANI/carbon cloth nanocomposites by interfacial polymerization with area-normalized capacitance of 1.8 F cm−2 at a discharge rate of 1.73 A g−1.14 Bian et al. explored a solid-state electrode with self-doped polyaniline on functionalized carbon, which showed a specific capacitance of 408 F g−1 in 0.5 M Na2SO4 at a current density of 1 A g−1.15 Zhou et al. fabricated activated polyaniline-based carbon nanoparticles with a specific capacitance of 341 F g−1.16 He et al. produced a polyaniline-coated electro-etched carbon fiber cloth electrode, wherein the polyaniline nanowires could achieve a mass-normalized specific capacitance of 673 F g−1 and an area-normalized capacitance of 3.5 F cm−2.17 However, few publications have reported a large gravimetric capacitance and a high surface capacitance.
Carbon fiber cloth (CFC) is a highly conductive and inexpensive textile with excellent mechanical strength and flexibility.18–20 CFC is also found to be a good 3D conducting framework, as both a high surface support for conducting polymers and a current collector.21–24 One of the advantages of these types of hybrid materials is that they do not need any binder or conductive agent (e.g., polyvinylidene fluoride or acetylene black) during preparation of the electrodes.25–27 However, limited reports focus on novel bearded carbon fiber cloth. The development of conductive polymer-modified novel bearded CFC as a flexible electrode to achieve high-performance energy-storage systems is strongly desired.
In this study, the pinhole PANI was decorated on novel bearded CFC, which offers additional electrical conductivity between electrode material and electrolyte. The unique porous structural characteristic of PANI not only provides a wide area for proton exchange (electron exchange) but also shortens the path length of ion transport. The composite electrodes were optimized to achieve a high area-normalized capacitance of 3.3 F cm−2 at current density of 1.3 A g−1. The large capacitance and voltage region allow high energy and power densities (36.35 Wh kg−1 and 422.15 W kg−1). The results showed that the combination of porous PANI with bearded CFC significantly increased the capacitance of the electrode and enhanced its electrochemical performance.
2. Experimental
2.1. Materials
Carbon fiber cloth (HCP330: plain-weave, 165 g m−2, 0.36 mm thickness; no wet-proofing) was purchased from Hesen Co., Ltd (Shanghai, China). Carbon cloth sheets with dimensions of 10 mm × 20 mm were treated in 5% potassium permanganate for 60 min, cleaned in alcohol and DI water several times, and dried at 70 °C for 6 h. The bearded CFC increased the surface area of the carbon fiber. A stainless steel coupon (SS, 25 cm2) was treated in sulfuric acid.2.2. Preparation of polyaniline/carbon fiber cloth (PANI/CFC)
The electrode sheet of PANI/CFC composite electrode was prepared as follows. 2.79 g aniline was infused into 200 mL (1 M) sulfuric acid with magnetic stirring for 10 minutes. A preliminary carbon cloth fiber sheet, silver (Ag)/silver chloride (AgCl), and 20 mm × 20 mm platinum sheet used as working, reference, and counter electrodes, respectively. The electrolyte composed of an aqueous solution of 0.15 M aniline and 0.1 M sulfuric acid was used for electro-polymerization. PANI thin films were electro-polymerized by sweeping the potential between −0.4 V and +1.3 V versus Ag/AgCl reference for 10 scans (scan rate: 20 mV s−1). All the experiments were performed at room temperature without stirring. Furthermore, the products were washed with distilled water and ethanol for three times, and dried in a vacuum at 70 °C for 6 h, and then, various properties were studied. Cyclic voltammetry was carried out using potentio-/galvanostat, an AUTOLAB PGSTAT30 (Metrohm, USA).2.3. Characterization
The morphologies of the PANI/CFC were characterized by transmission electron microscopy (TEM, JEM-2100, JEOL, Japan) at an accelerating voltage of 200 kV. Scanning electron microscopy (SEM, JSM-6510, JEOL, Japan) and energy dispersive X-ray spectroscopy (EDS) were performed on the same SEM microscope and field-emission scanning electron microscopy (FE-SEM, JSM-6700F, JEOL, Japan) was performed at 5 kV. FTIR spectra were obtained on a Spectrum-GX Perkin Elmer spectrometer.A three-electrode system was used in electrochemical experiments on an AUTOLAB electrochemical workstation, where PANI/CFC (1.0 cm × 1.0 cm) was used as the working electrode, silver/silver chloride (SCE) was used as the reference electrode, a platinum sheet was used as the counter electrode, and 1 M H2SO4 was used as the electrolyte. Cyclic voltammetry (CV) was carried out in a potential range from −0.2 to 1.0 V (vs. SCE) at scan rates of 10, 20, 40, 80, and 100 mV s−1. Galvanostatic charge/discharge properties were measured at step-increasing current densities of 2–20 mA cm−2 from 0 to 1 V. The cycle performance of the composite electrode of PANI/CFC was tested using the battery test system (Land Co., Ltd, Wuhan, China).
3. Results and discussion
We used an efficient and facile method to synthesize the porous and ordered nanostructure of PANI based on CFC with a novel beard, as shown in Fig. 1. PANI/CFC composite electrode was prepared by two-step: first, CFC was pretreated and then was used for electrochemical deposition of porous PANI.3.1. Morphology and structure
Fig. 2 presents the SEM images of CFC before and after oxidation. The CFC sheet with beard surface is observed, about 8.5 ± 1.5 μm in diameter. These beards were fast in electron transportation of the electrochemical capacitor. After oxidation, shown in Fig. 2b, the surface area was enlarged; nitrogen adsorption results were used to give further evidence of it. The initial CFC had an area of 55.26 m2 g−1, whereas after oxidation, the specific area was increased to 450.97 m2 g−1. It was easy to deposit PANI on CFC through the pretreatment process.The FE-SEM image (Fig. 2c) of CFC with PANI layers electrodeposited by 5 scans revealed that the beards were free-standing as before. To investigate the beard-like carbon fiber, the original CFC was cut into several pieces, sonicated in a solution, and then transferred onto a holey-carbon copper grid for TEM studies. The TEM of beard-like carbon fiber (Fig. 2c inset) supported the fact that free-standing beard-like structures of carbon fiber, with 40 nm in diameter, are formed.
The SEM of PANI/CFC composite electrode in Fig. 2d revealed that porous PANI was deposited on the carbon fiber rods skeleton, showing the microstructures of carbon fiber enwrapped with a PANI layer deposited with 10 scans, which displayed an efficient bonding between CFC and PANI. During the polymerization of PANI, aniline monomers are adsorbed on surfaces of the CFC via π–π conjugation and chemical bonding effects. The adsorbed aniline molecules act as nucleation sites and react with adjacent aniline molecules to form PANI films. The PANI/CFC had an area of 286.12 m2 g−1. The diameter of carbon fiber was about 11 μm, and it was increased by 3 μm from the original diameter. Such a microstructure of PANI can be used for high performance electrochemical capacitors and its porous structure was the main reason that the composite electrode provided significant enhancement with a redox-active surface area. Moreover, this indicates that a fully intact interface between PANI and CFC improves the charge transport.28
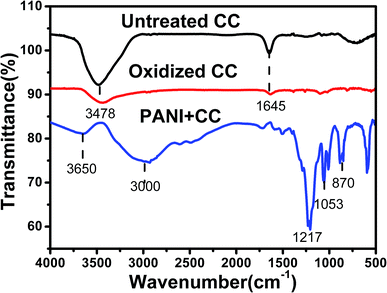
Fourier transform infrared (FTIR) spectroscopy was employed to further confirm the surface properties and chemical composition of the obtained samples (Fig. 3). For pure and oxidized CFC, the broad peak at about 3478 cm−1 can be allocated to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching. Furthermore, an O–H deformation vibration near 1645 cm−1 was found for pure and oxidized CFC. For the PANI/CFC composite electrode, the bands at around 3650 cm−1 and 3000 cm−1 were attributed to –NH and CH stretching, which show typical PANI vibrations. The bands at 1217 and 1053 cm−1 are the stretching vibrations of C–N in aromatic amines and CN˙+, which shows the polaron structure of PANI.29 In the PANI/CFC composite electrode, the deprotonated sulfonic acid group doped in PANI acted as a counter anion. The negative charge on the sulfonate group is ascribed to the enhancement of O
O stretching. Furthermore, an O–H deformation vibration near 1645 cm−1 was found for pure and oxidized CFC. For the PANI/CFC composite electrode, the bands at around 3650 cm−1 and 3000 cm−1 were attributed to –NH and CH stretching, which show typical PANI vibrations. The bands at 1217 and 1053 cm−1 are the stretching vibrations of C–N in aromatic amines and CN˙+, which shows the polaron structure of PANI.29 In the PANI/CFC composite electrode, the deprotonated sulfonic acid group doped in PANI acted as a counter anion. The negative charge on the sulfonate group is ascribed to the enhancement of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–S bonds and the blue-shift of the vibrations. The sulfonate groups were attached to the polymer chains of PANI. For the vibration of O
O and C–S bonds and the blue-shift of the vibrations. The sulfonate groups were attached to the polymer chains of PANI. For the vibration of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, less blue-shifted, the negative charges can be partly delocalized on the conjugated polymer chain.
O, less blue-shifted, the negative charges can be partly delocalized on the conjugated polymer chain.
The elemental compositions of the samples were measured by SEM–EDS, as tabulated in Table 1. Comparing oxidized CFC with pure CFC, there was a decrease in the C content and an increase in O content; Mn and S were introduced during the synthesis process, which might be due to oxidation during pre-treatment.
| Sample | C | N | O | Mn | S |
|---|---|---|---|---|---|
| Untreated CFC | 96.54 | 0 | 2.99 | 0 | 0 |
| Oxidized CFC | 79.61 | 0 | 9.09 | 9.77 | 0.40 |
| PANI/CFC | 55.27 | 6.98 | 27.75 | 0 | 10.00 |
Comparing PANI/CFC with oxidized CFC, there was a decrease in the C content and an increase in O; S and N were introduced during the synthesis process, which might be due to the doping of PANI with SO42−, as it was electro-polymerized in 1 M H2SO4 solution.
3.2. Electrochemical property
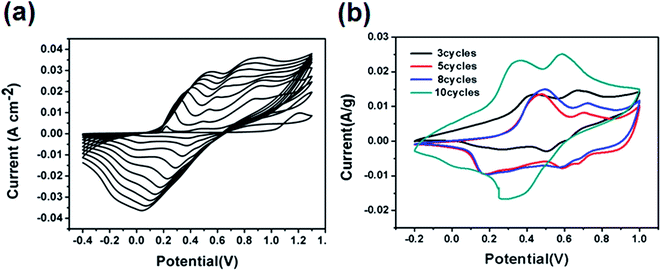
The CV curves of the electrodeposition process on the SS from 1 to 10 cycles are shown in Fig. 4a, where two anodic peaks and two cathodic peaks are observed. With successive potential scans, the peak currents increased persistently, indicating that the deposition of conducting PANI on the stainless steel substrate has indeed been achieved, and we can clearly see the green film on the SS sheet.Fig. 4b shows the CV curves of the electrochemical deposition of PANI on CFC with different cycles of 3, 5, 8 and 10 at the scan rate of 20 mV s−1. There were two pairs of oxidation–reduction peaks, the first pair of peaks was due to the PANI structure transitions of the redox to emeraldine, and the second pair of peaks was due to the PANI structure transitions of emeraldine to pernigraniline. With the increase in scanning cycles, the area of the CV curve increased correspondingly. These results suggested that the content of PANI as the main active component increased with the number of deposition cycles. Note that the PANI/CFC composite electrode with high PANI content was very susceptible to fall-off after subsequent cycles, as demonstrated by the CV curves at different cycle numbers. The PANI/CFC composite electrode of 10 cycles thus showed the value that provided the best balance to ensure a continuous, defectless and flexible film bonding with a high specific capacitance.
We executed a combination of measurements: electrochemical impedance spectroscopy, cyclic voltammetry and galvanostatic charge–discharge. The impedance curve of PANI/CFC-based electrodes measured in 1 M H2SO4 electrolyte is shown in Fig. 5a. Ideal capacitive behavior of the near-vertical shape curve obtained at lower frequencies was observed. The equivalent series resistance (ESR) extracted at high frequency (100 kHz) was about 2.7 Ω. In addition, the charge transfer resistance read from the small size of the semicircle (<0.32 Ω) was very small, suggesting good ion transport in the continuous three-dimensional nanostructure frame. The rate-dependent cyclic voltammogram curve with a potential window from −0.2 to 1.0 V vs. Ag/AgCl reference electrode were scanned at rates of 10, 20, 40, 80, and 100 mV s−1, as shown in Fig. 5b. The typical PANI redox peaks were clearly seen in the cyclic voltammetry curve. Galvanostatic charge–discharge measurements were made at different current densities. Fig. 5c presents the discharge curve of the electrode at current densities of 2, 5, 10, 15 and 20 mA cm−2.
Their specific capacitances (Cm) can be calculated with eqn (1)
 | (1) |
3.3. Study of the asymmetric supercapacitor devices
Although there are many reports about PANI-based composite electrode materials, reports of flexible supercapacitors assembled with electrochemical deposition of PANI composites are limited. Fig. 6a shows a schematic of the sandwich structure of a two-electrode supercapacitor and the separator between asymmetric electrodes. To assemble an asymmetric supercapacitor, CFC/PANI composite and the activated carbon fiber cloth (CFC) were used as the positive and negative electrodes, respectively. The positive electrode mass was 49.5 mg and the negative electrode mass was 83.2 mg. A thin layer of Nafion 117 was used as the separator. Two short pieces of copper foil pressed on the ends of the CFC served as connectors. The image of the flexible all-solid-state capacitor is shown in Fig. 6b. A flexible all-solid-state capacitor was assembled with H2SO4-Nafion as the separator between asymmetric electrodes. | ||
| Fig. 6 (a) Schematic of the all-solid-state capacitor with a flexible Cu foil as the substrate. (b) Image of the flexible capacitor. | ||
The cyclic voltammetry curves of the capacitor (Fig. 7a) showed the shape of pseudocapacitive behavior and the curve was conservative even at high scan rates. The charge–discharge curves of the flexible capacitor at different current densities are shown in Fig. 7b. At a current density of 2 mA cm−2, the special capacitance of the device can reach up to 28.4 F g−1. In order to evaluate the performance of the all-solid-state asymmetric hybrid capacitors for flexible energy storage systems, a device placed under different bending conditions is shown in Fig. S1.† A digital image showing the flexibility of the device is shown in Fig. S1(a),† and the asymmetric hybrid capacitors can be bent from 0° to 180°, as shown in Fig. S1(b),† without degrading their performance, which indicates their high flexibility, as seen in those CV curves collected at a scan rate of 20 mV s−1.
 | ||
| Fig. 7 (a) CV curves of the flexible capacitor at various scan rates (10–100 mV s−1). (b) Charge–discharge curves of the flexible capacitor at different current densities (2–5 mA cm−2). | ||
Energy density (E) and power density (P) of the capacitors can be calculated from eqn (2) and (3), respectively, where Cs is the specific capacitance calculated in F g−1 from charge–discharge curve, ΔV is the potential drop in V during the discharge curve, E is the energy density of the electrode in Wh kg−1, and t was the discharge time in h. PANI/CFC has the highest E at higher P. From the abovementioned calculation, a maximum power density of 422.15 W kg−1 is obtained for the PANI/CFC capacitors at a specific energy of 36.35 Wh kg−1.
 | (2) |
 | (3) |
Table 2 compares our supercapacitor with previously reported similar PANI-based composite electrodes and flexible capacitors. The energy density of the asymmetric supercapacitor is 36.35 Wh kg−1, which is higher than that observed with flexible capacitors based on composites of PANI and functionalized carbon cloth (9.3 Wh kg−1).15 To the best of our knowledge, this value of specific energy is very close to the highest so far reported for PANI.31 This result demonstrates that the porous structure can increase the utilization of PANI greatly.32 In addition, the capacitor also exhibited good cycling stability and the corresponding data are shown in Fig. S2.† During charging and discharging processes, due to swelling and shrinking of conducting polymers, electro-active polymer-based supercapacitors often suffer from limited cycling performance.33–36 The cyclability of our nanostructure composite electrodes was as high as ∼76% capacitance retention over 1000 cycles at the current density of 5 A g−1. Charge–discharge curves of the final five cycles are presented in the inset.
| Composites | Electrolyte | Electrode/F g−1 | Capacitor/F g−1 | Energy density/Wh kg−1 | Power density/W kg−1 | Reference |
|---|---|---|---|---|---|---|
| a FC: functionalized carbon cloth, AC: activated carbon, DMCX: order mesoporous carbon, NG-PAA: nitrogen-doped graphene-enhanced polyacrylic acid, CNT: carbon nanofiber, ACF: activated carbon fibers. | ||||||
| CFC/PANI | 1 M H2SO4 | 808 | 28.4 | 36.35 | 422.15 | This work |
| FC/PANI | 2 M H2SO4 | 408 | 67 | 9.3 | — | 15 |
| AC/PANI | 1 M H2SO4 | 338 | — | — | — | 24 |
| DMCX/PANI | 0.5 M H2SO4 | 343 | — | 6.57 | 1000 | 33 |
| NG-PAA/PANI/CC | 1 M H2SO4 | 521 | 68 | 5.8 | 1100 | 34 |
| CNT/PANI | 0.5 M H2SO4 | 16 | — | 0.5 | 300 | 35 |
| ACF/PANI | 1 M H2SO4 | 61 | 55.3 | 20 | 2100 | 36 |
Another performance index was the volumetric energy density, which was a key parameter for most applications. The paper-based asymmetric device exhibited a volume capacitance of 11 F cm−3 at a current density of 2 mA cm−3, and an energy density of about 0.96 mWh cm−3 at a power density of 0.162 W cm−3 normalized to the whole volume of the solid-state device.37–41
In view of the results, the porous PANI coating of a novel bearded carbon fiber cloth enhanced the specific energy density of the asymmetric devices, due to its doping/de-doping processes that increase the capacitance of the positive electrode and the large potential window.
4. Conclusions
In summary, a facile and effective method for the preparation of high porosity and three-dimensional conductive polymers by electro-polymerization was presented. Our PANI/CFC electrodes generated excellent rate performance, only ∼7% capacitance loss when the current density increased by a factor of 15.4, and showed better performance with higher specific capacitance and good stability. The unique pinhole structural characteristics of PANI shorten the path length for electrolyte ion transport and provide a bigger contact surface area for proton interactions. The optimal composite electrodes achieved a high gravimetric capacitance of 808 F g−1 and a high area-normalized capacitance of 3.3 F cm−2. The results showed that the electrodeposition of polyaniline on CFC notably enhanced the electrochemical performance of the electrodes.The asymmetric supercapacitor was assembled using the composite CFC/PANI prepared by electrochemical deposition as the positive electrode and CFC as the negative electrode. This capacitor was tested in a cell voltage of 0–1.6 V and exhibited high energy densities of 36.35 Wh kg−1, calculated for the unpacked active materials, and power densities of 422.15 W kg−1.
Acknowledgements
This study was jointly supported by the Qing Lan Project, the Innovation Fund of Jiangsu Province (No. BY2013072-06), the Advanced Access Engineers for Higher Vocational Colleges Teachers of Jiangsu Province (2015FG032), the Natural Science Foundation of Jiangsu Province (No. 20161289), the Research Innovation Program for College Graduates and Students of Jiangsu Province (KYZZ15_0043) and the National Natural Science Foundation of China (No. 11374136).References
- B. D. Gate, Flexible electronics, Science, 2009, 323, 1566–1567 CrossRef PubMed.
- F. Meng and Y. Ding, Sub-Micrometer-Thick All-Solid-State Supercapacitors with High Power and Energy Densities, Adv. Mater., 2011, 23, 4098–4102 CrossRef CAS PubMed.
- X. Li, X. Zang, Z. Li, X. M. Li, P. X. Li, P. Z. Sun, X. Lee, R. J. Zhang, Z. H. Huang, K. L. Wang, D. H. Wu, F. Kang and H. Zhu, Large-Area Flexible Core–Shell Graphene/Porous Carbon Woven Fabric Films for Fiber Supercapacitor Electrodes, Adv. Funct. Mater., 2013, 23, 4862–4869 CAS.
- L. J. Pan, G. H. Yu, D. Zhai, H. Lee, W. Zhao, N. Liu, H. Wang, B. Tee, Y. Shi, Y. Cui and Z. N. Bao, Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9287–9292 CrossRef CAS PubMed.
- A. C. Nwanya, C. J. Jafta, P. M. Ejikeme, P. E. Ugwuoke, M. V. Reddy, R. U. Osuji, K. I. Ozoemena and F. I. Ezema, Electrochromic and electrochemical capacitive properties of tungsten oxide and its polyaniline nanocomposite films obtained by chemical bath deposition method, Electrochim. Acta, 2014, 128, 218–225 CrossRef CAS.
- Q. Q. Zhang, Y. Li, Y. Y. Feng and W. Feng, Electropolymerization of graphene oxide/polyaniline composite for high-performance supercapacitor, Electrochim. Acta, 2013, 90, 95–100 CrossRef CAS.
- E. Karaca, N. Ö. Pekmez and K. Pekmez, Galvanostatic deposition of polypyrrole in the presence of tartaric acid for electrochemical supercapacitor, Electrochim. Acta, 2014, 147, 545–556 CrossRef CAS.
- J. Ma, H. J. Ni, D. Y. Su, M. Y. Huang and X. X. Wang, The research status of Nafion ternary composite membrane, Int. J. Hydrogen Energy, 2012, 37, 13185–13190 CrossRef CAS.
- E. Mitchell, J. Candler, F. De Souza, R. K. Gupta, B. K. Gupta and L. F. Dong, High performance supercapacitor based on multilayer of polyaniline and graphene oxide, Synth. Met., 2015, 199, 214–218 CrossRef CAS.
- H. Y. Jin, L. M. Zhou, C. L. Mak, H. T. Huang, W. M. Tang and H. L. W. Chan, High-performance fiber-shaped supercapacitors using carbon fiber thread (CFT)@polyanilne and functionalized CFT electrodes for wearable/stretchable electronics, Nano Energy, 2015, 11, 662–670 CrossRef CAS.
- C. W. Kuo, C. C. Yang and T. Y. Wu, Facile Synthesis of Composite Electrodes Containing Platinum Particles Distributed in Nanowires of Polyaniline-Poly(Acrylic Acid) for Methanol Oxidation, Int. J. Electrochem. Sci., 2011, 6, 3196–3209 CAS.
- S. Q. Jiao, J. G. Tu, C. Y. Fan, J. G. Hou and D. J. Fray, Electrochemically assembling of a porous nano-polyaniline network in a reverse micelle and its application in a supercapacitor, J. Mater. Chem., 2011, 21, 9027–9030 RSC.
- Z. B. Lei, Z. W. Chen and X. S. Zhao, Growth of Polyaniline on Hollow Carbon Spheres for Enhancing Electrocapacitance, J. Phys. Chem. C, 2010, 114, 19867–19874 CAS.
- Y. Y. Horng, Y. C. Lu, Y. K. Hsu, C. C. Chen, L. C. Chen and K. H. Chen, Flexible supercapacitor based on polyaniline nanowires/carbon cloth with both high gravimetric and area-normalized capacitance, J. Power Sources, 2010, 195, 4418–4422 CrossRef CAS.
- L. J. Bian, F. Luan, S. S. Liu and X. X. Liu, Self-doped polyaniline on functionalized carbon cloth as electroactive materials for supercapacitor, Electrochim. Acta, 2012, 64, 17–22 CrossRef CAS.
- J. Zhou, T. T. Zhu, W. Xing, Z. H. Li, H. L. Shen and S. P. Zhuo, Activated polyaniline-based carbon nanoparticles for high performance supercapacitors, Electrochim. Acta, 2015, 160, 152–159 CrossRef CAS.
- X. P. He, B. Gao, G. B. Wang, J. T. Wei and C. Zhao, A new nanocomposite: Carbon cloth based polyaniline for an electrochemical supercapacitor, Electrochim. Acta, 2013, 111, 210–215 CrossRef CAS.
- A. B. Rohom, P. U. Londhe, S. K. Mahapatra, S. K. Kulkarni and N. B. Chaure, Electropolymerization of polyaniline thin films, High Perform. Polym., 2014, 26, 641–646 CrossRef.
- X. B. Zang, X. Li, M. Zhu, X. M. Li, Z. Zhen, Y. J. He, K. L. Wang, J. Q. Wei, F. Y. Kang and H. W. Zhu, Graphene/polyaniline woven fabric composite films as flexible supercapacitor electrodes, Nanoscale, 2015, 7, 7318–7322 RSC.
- S. K. Mondal, K. Barai and N. Munichandraiah, High capacitance properties of polyaniline by electrochemical deposition on a porous carbon substrate, Electrochim. Acta, 2007, 52, 3258–3264 CrossRef CAS.
- V. Gupta and N. Miura, Electrochemically Deposited Polyaniline Nanowire's Network A High-Performance Electrode Material for Redox Supercapacitor, Electrochem. Solid-State Lett., 2005, 12, A630–A632 CrossRef.
- E. Detsi, P. Onck and J. T. M. D. Hosson, Metallic Muscles at Work: High Rate Actuation in Nanoporous Gold/Polyaniline Composites, ACS Nano, 2013, 7, 4299–4306 CrossRef CAS PubMed.
- S. W. Zhang, M. Y. Zeng, W. Q. Xu, J. X. Li, J. Li, J. Z. Xu, X. K. Wang, Polyaniline nanorods dotted on graphene oxide nanosheets as a novel super adsorbent for Cr(VI), The Royal Society of Chemistry, 2013, vol. 42, pp. 7854–7858 Search PubMed.
- A. Olad and H. Gharekhani, Preparation and electrochemical investigation of the polyaniline/activated carbon nanocomposite for supercapacitor applications, Prog. Org. Coat., 2015, 81, 19–26 CrossRef CAS.
- G. M. Wang, H. Y. Wang, X. H. Lu, Y. C. Ling, M. H. Yu, T. Zhai, Y. X. Tong and Y. Li, Solid-State Supercapacitor Based on Activated Carbon Cloths Exhibits Excellent Rate Capability, Adv. Mater., 2014, 26, 2676–2682 CrossRef CAS PubMed.
- A. K. Mishra and S. Ramaprabhu, Functionalized graphene-based nanocomposites for supercapacitor application, J. Phys. Chem. C, 2011, 115, 14006–14013 CAS.
- P. P. Yu, Y. Z. Li, X. Y. Yu, X. Zhao, L. H. Wu and Q. H. Zhang, Polyaniline Nanowire Arrays Aligned on Nitrogen-Doped Carbonn Fabric for High-Performance Flexible Supercapacitors, Langmuir, 2013, 29, 12051–12058 CrossRef CAS PubMed.
- P. J. Peerce and A. J. Bard, Polymer films on electrodes: Part II. Film structure and mechanism of electron transfer with electrodeposited poly(vinylferrocene), J. Electroanal. Chem. Interfacial Electrochem., 1980, 112, 97–115 CrossRef CAS.
- T. Gueshi, K. Tokuda and H. Matsuda, Voltammetry at partially covered electrodes: Part II. Linear potential sweep and cyclic voltammetry, J. Electroanal. Chem. Interfacial Electrochem., 1979, 101, 29–38 CrossRef CAS.
- G. P. Wang, L. Zhang and J. J. Zhang, A review of electrode materials for electrochemical supercapacitors, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- M. A. Bavio, G. G. Acosta and T. Kessler, Energy storage in symmetric and asymmetric supercapacitors base in carbon cloth/polyaniline-carbon black nanocomposites, Int. J. Energy Res., 2015, 39, 2053–2061 CrossRef CAS.
- G. H. Yu, X. Xie, L. J. Pan, Z. N. Bao and Y. Cui, Hybrid nanostructured materials for high-performance electrochemical capacitors, Nano Energy, 2013, 2, 213–234 CrossRef CAS.
- Z. S. Zhang, G. Wang, Y. Li, X. Zhang, N. L. Qiao and J. H. Wang, et al., A new type of ordered mesoporous carbon/polyaniline composites prepared by a two-step nanocasting method for high performance supercapacitor applications, J. Mater. Chem. A, 2014, 2, 16715–16722 CAS.
- Y. G. Wang, S. C. Tang, S. Vongehr, J. Ali Syed, X. Y. Wang and X. K. Meng, High-Performance Flexible Solid-State Carbon Cloth Supercapacitors Based on Highly Processible N-Graphene Doped Polyacrylic Acid/Polyaniline Composites, Sci. Rep., 2016, 6 DOI:10.1038/srep12883.
- Q. Liu, M. H. Nayfeh and S. T. Yau, Brushed-on flexible supercapacitor sheets using a nanocomposite of polyaniline and carbon nanotubes, J. Power Sources, 2010, 195, 7480–7483 CrossRef CAS.
- D. Salinas-Torres, J. M. Sieben, D. Lozano-Castelló, D. Cazorla-Amorós and E. Morallón, Asymmetric hybrid capacitors based on activated carbon and activated carbon fiber-PANI electrodes, Electrochim. Acta, 2013, 89, 326–333 CrossRef CAS.
- Y. Gogotsi and P. Simon, True Performance Metrics in Electrochemical Energy Storage, Science, 2011, 334, 917–918 CrossRef CAS PubMed.
- Q. Wang, J. Yan and Z. J. Fan, Carbon materials for high volumetric performance supercapacitor: design, progress, challenges and opportunities, Energy Environ. Sci., 2016, 9, 729–762 CAS.
- J. Yan, Y. Xiao, G. Q. Ning, T. Wei and Z. J. Fan, Facile and rapid synthesis of highly crumpled graphene sheets as high-performance electrodes for supercapacitors, RSC Adv., 2013, 3, 2566–2571 RSC.
- J. Li, K. Liu, X. Gao, B. Yao, K. F. Huo, Y. L. Cheng, D. C. Chen, B. Wang, W. M. Sun, D. Ding, M. L. Liu and L. Huang, Oxygen- and Nitrogen-Enriched 3D Porous Carbon for Supercapacitors of Volumetric Capacity, ACS Appl. Mater. Interfaces, 2015, 7, 24622–24628 CAS.
- J. Wang, B. Ding, Y. L. Xu, L. F. Shen, H. Dou and X. G. Zhang, Crumpled Nitrogen-Doped Graphene for supercapcaitors with Gravimetric and Volumetric Performances, ACS Appl. Mater. Interfaces, 2015, 7, 22284–22291 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16291f |
| This journal is © The Royal Society of Chemistry 2016 |