DOI:
10.1039/C6RA16049B
(Paper)
RSC Adv., 2016,
6, 70547-70552
Nanosorbcats of methylene blue on novel Fe2O3 nanorods for photocatalytic water oxidation†
Received
21st June 2016
, Accepted 9th July 2016
First published on 13th July 2016
Abstract
The development of dye-loaded nanosorbcats can make best use of their advantages in removing dyes from wastewater, also bypassing their disadvantages. Herein, functionalized Fe2O3 nano-rods (pfa@Fe2O3) were synthesized by using tween-80 induced assembly of pyrrol-2-methanoic acid (pfa) and iron(III) salts, and characterized by FT-IR, XRD, EPR, and SEM. The pfa@Fe2O3 nanorods’ adsorption capacity for methylene blue (MB) was 450 mg g−1 compared to only 39 mg g−1 for α-Fe2O3. Moreover, the kinetics to reach equilibrium were approximately 0.5 h and the pfa@Fe2O3 nanorods can be used as efficient recyclable adsorbents for MB. In particular, the MB loaded MB@pfa@Fe2O3–water system could also be used as dioxygen generator when irradiated with red LED light (10 W). The light induced intramolecular charge transfer between catalysts and photosensitizers was more efficient in the MB@pfa@Fe2O3/H2O system, and it exerted higher activity for water oxidation than the traditional catalyst/Ru(bpy)32+ system. Control experiment results suggested that the α-Fe2O3 can’t be used as LED light driven catalysts for water oxidation. In conclusion, the MB@pfa@Fe2O3 nanorods were efficient recyclable nanosorbcats for LED-light-driven dioxygen generation using water as oxygen source.
Introduction
Effluents of colored wastewater from the textile industry contain many toxic and organic dyes which can cause severe environmental damage that affects aquatic ecosystems. Dyes are also known to be carcinogenic and toxic, thus causing serious environmental problems that are mutagenic for aquatic organisms.1 The removal of dyes from the colored effluent has therefore attracted increasing research attention.2 Physical and chemical technologies such as adsorption,3–6 membrane separation,7–9 catalytic oxidation,10,11 etc. have been reported to date. Among these, the adsorption techniques have proved to be an effective process for removal of non-biodegradable dyes from wastewater. High sludge production and post-treatment of toxic sludge are major problems in large-scale applications.12,13 In addition, catalytic oxidation generates highly reactive radicals that drive degeneration of new discolored species in the wastewater. There are some problems in terms of recovery of catalysts from the wastewater and high cost due to usage of large amounts of oxidants. Moreover, the toxicity of the discolored species to aquatic organisms is another problem. From the view of environmental effects iron oxide nanoparticles are considered to be naturally occurring and their applications for wastewater treatment have focused on using them as adsorbents for removal of contaminants or as catalysts for photocatalytic degradation of contaminants.14,15 For example, cysteamine modified iron oxide nanoparticles were able to degrade a model organic dye in water.16 Also, groundwater treatment sludge was transferred to magnetic particles for the adsorption of methylene blue.17 Fe2O3 nanoparticles have recently been reported as nanoadsorbents and catalysts (nanosorbcats) for methylene blue (MB) adsorption and oxidation.18
Water oxidation catalysts (WOCs) based on iron are highly attractive due to their abundance, greenness, and low-cost. Most of the iron(III) based complexes have been reported as catalysts for water oxidation at low pH using CeIV and IO4− as oxidants,19,20 and also as light-driven water oxidation catalysts under basic conditions in the presence of Ru(bpy)32+ (bpy = 2,2′-bipyridine).21–23 The development of dye-loaded nanosorbcats can make the best use of the advantages and disadvantages of the removal of dyes from the wastewater. However, research on the dye-loaded iron oxide nanoparticles (as nanosorbcats) for water oxidation has been rarely reported. We previously found that the cobalt(II) complex of 1H-pyrrol-2-yl-methanone (PPM) could catalyze water oxidation without extra electron accepters.24 We therefore proposed that 1H-pyrrol-2-methanic acid assembling with nano iron(III) oxide may lead to new types of light-driven super-molecular nanoparticles for efficient dye adsorption and formation of nanosorbcats for LED light driven dioxygen generation using water as oxygen source. Herein, we report the synthesis of economic iron oxide nanorods as effective adsorbents of dyes (MB). Regeneration of the adsorbent was easily carried out by an extraction process. In particular, the MB-loaded nanoadsorbents were subsequently upgraded as valuable recyclable catalysts (nanosorbcats) for the LED-light-driven water oxidation reaction.
Materials and methods
Materials
Pyrrol-2-methanoic acid (pfa) and methylene blue (MB) were used as analytical grade reagents (Aladdin Reagent Co. Ltd., Shanghai) without further purification. α-Fe2O3 was synthesized as reported.25 All the other chemicals used for synthesis were analytical grade reagents (Sinopharm Chemical Reagent Co. Ltd., China), used without further purification. Water was purified with a Millipore Milli-Q system.
Characterization
The IR spectra were recorded on a Niconet-470 spectrophotometer in the range of 4000–400 cm−1. The electronic absorption spectra were recorded in the 200–800 nm regions using a Varian Cary 50-Bio UV-vis spectrophotometer. SEM was carried out on a JEOL JSM-7001F field emission scanning electron microscope at an acceleration voltage of 15 kV. The content of iron in the nanoparticles was determined using an ICP-AES instrument (HORIBA Jobin Yvon, Longjumeau Cedex, France). X-Band EPR spectra were recorded on a Bruker Biospin GmbH instrument at room temperature.
Synthesis of pfa@Fe2O3
Ferric chloride hexahydrate aqueous solution (0.25 M, 20 ml) was added into the pyrrol-2-methanoic acid (pfa) aqueous solution (0.1 M, 50 ml) with Tween-80 (5% v/v). The mixture was stirred for 15 min under room temperature, followed by its transfer into a reaction kettle and further heating for 6 h at 160 °C. After removal of the solvent by centrifugation, the product was washed with ethanol three times and dried in vacuum to give a black solid product. The yield was as follows: 892 mg (83%). IR (KBr, cm−1): 3263, 2956, 2924, 2853, 1697.
Adsorption experiments
Adsorption experiments for MB using α-Fe2O3 and pfa@Fe2O3 were carried out by mixing 10.0 mg of α-Fe2O3 and pfa@Fe2O3 in MB solution (10.0 ml). After the mixture was stirred for some time, the mixture was separated and analyzed. The absorption of MB solutions was measured by UV-vis absorption spectra to monitor absorption at λmax = 664 nm. The concentration of MB solution was determined by linear regression equation obtained by plotting the calibration curve for MB over a range of concentrations. Adsorption efficiency for MB by α-Fe2O3 or pfa@Fe2O3 was calculated using the following expression:
where qt was the MB (mg l−1) adsorption quantity at t min. C0 and Ct were the initial concentrations of MB (mg l−1) in the solution and t min, respectively.
Water oxidation experiments
Water oxidation experiments, catalyzed by MB loaded pfa@Fe2O3 (MB@pfa@Fe2O3) or pfa@Fe2O3/RuII(bpy)32+, were carried out by mixing the MB@pfa@Fe2O3 (or pfa@Fe2O3/RuII(bpy)32+) with water at 298 K under red (or blue) LED light (10 W) irradiation. The MB@pfa@Fe2O3 nanorods were prepared by adding pfa@Fe2O3 into the MB solutions for some time and then the product were separated and dried in an oven. The volume of O2 was measured by direct methods via connection of the reaction vessel with a U-tube to a calibrated microburet to collect the released gas. Oxygen evolution was monitored by gas chromatography using a thermal conductivity detector (GC-TCD).
Result and discussion
Synthesis and characterization of pfa@Fe2O3
The pfa@Fe2O3 nanorods were synthesized using surfactant template (tween-80) induced assembly of the pyrrol-2-methanoic acid (pfa) and iron(III) salts by hydrothermal reaction at 433 K. The stretching frequency of –COOH (approximately 1706 cm−1) in the IR spectrum of pfa@Fe2O3 (Fig. 1) indicated the existence of a pyrrol-conjugated carboxyl group, which shifted from 1720 cm−1 to 1706 cm−1 (free –COOH) due to coordination of carboxylic acid and iron(III) atoms. The main peaks at 228, 285, 400, 636 and 1336 cm−1 in the resonance Raman spectra of pfa@Fe2O3 (Fig. 2) were assigned to α-Fe2O3,16 which was very similar to pure α-Fe2O3. In addition, the peak at 1571 cm−1 was assigned to Fe–OOC–, indicating the coordination of pyrrol-2-methanoic acid with iron(III) atom. Moreover, these results were entirely consistent with those from the X-ray diffraction spectrum (XRD) analysis of pfa@Fe2O3 (Fig. 3). The diffraction peaks at 2θ = 24°, 33°, 36°, 41°, 43°, 49°, 54°, 58°, 62°, 64°, 72°, and 77° were assigned to α-Fe2O3, indicating that the main structure of pfa@Fe2O3 was similar to α-Fe2O3. The XRD pattern displayed broad peaks at around 2θ = 25° at the same time, which was due to the diffraction of amorphous pyrrol-2-methanoic acid. These results revealed that the pfa@Fe2O3 had been successfully synthesized. Furthermore, the EPR spectra for the pfa@Fe2O3 nanorods (Fig. 4) showed a peak at g = 4.3, which was attributed to rhombic high-spin Fe(III) species, while the peak at g = 2.0 was more consistent with an organic radical.26 The SEM image (Fig. 5) indicated that the pfa@Fe2O3 nanoparticles were rod-like in shape. Based on the above results, it can be concluded that the synthesized pfa@Fe2O3 were rhombic rod-like Fe2O3 nanoparticles in which the iron(III) was coordinated with pyrrol-2-methanoic acid.
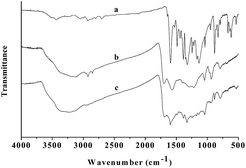 |
| | Fig. 1 (a) FT-IR spectrum of MB; (b) FT-IR spectrum of pfa@Fe2O3; (c) FT-IR spectrum of MB@ pfa@Fe2O3. | |
 |
| | Fig. 2 Resonance Raman spectrum of pfa@Fe2O3. | |
 |
| | Fig. 3 X-Ray diffraction of pfa@Fe2O3. | |
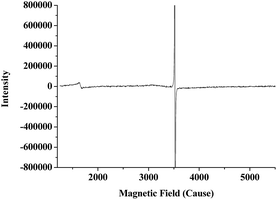 |
| | Fig. 4 X-Band EPR spectrum of pfa@Fe2O3. Experimental conditions: frequency: 9.851 GHz, power: 1.185 mW, modulation frequency: 100 kHz, modulation amplitude: 1 G, time constant: 20.480 ms. | |
 |
| | Fig. 5 SEM image of pfa@Fe2O3. | |
Adsorption capacity of pfa@Fe2O3 to MB
The adsorption capacity of pfa@Fe2O3 was compared with that of α-Fe2O3. It was obviously found that the adsorption capacity (MB/adsorbent) for the pfa@Fe2O3 (450 mg g−1) was approximately 12 times higher than that of the α-Fe2O3 (39 mg g−1). Moreover, the time required to reach the equilibrium was approximately 0.5 h (Fig. 6). The adsorbed amount of MB showed no obvious increase with increasing time, which was different from α-Fe2O3, in which the adsorbed amount of MB gradually increased with increasing time. The pfa@Fe2O3 also showed excellent removal capacity for MB in the solution, removing 98.4% of the MB (initial concentration, 100 mg l−1) from aqueous solution at the adsorbent dosage of 1000 mg l−1 (10 mg adsorbent in 10 ml MB solution), which was much higher than that of the α-Fe2O3 (only 19.5% for MB). As shown in Fig. 7, the adsorption capacity of pfa@Fe2O3 increased and was then constant with increasing concentration of the MB solution. The achieved adsorption capacity was approximately 450 mg g−1, thus indicating that the increase of the initial dye concentration contributed to enhancement of the driving force at the solid–liquid interface (ion exchange, electrostatic attraction and chemical association) until the adsorption sites were saturated. In addition, the adsorption capacity of pfa@Fe2O3 decreased with increasing amount of the pfa@Fe2O3, thus suggesting that the increase of the adsorbent may not have been conducive for the driving force at the solid–liquid interface until the adsorption sites were saturated. Moreover, compared to other reported adsorbents such as Fe2O3/CNT, Fe3O4/CNT and Fe/ordered mesoporous carbon,27 the pfa@Fe2O3 nanorods showed a relatively high adsorption capacity for adsorption of the MB. This indicated that the pfa@Fe2O3 nanorods were greatly superior to the α-Fe2O3, and could be used as an efficient and promising adsorbent for the decontamination of wastewater containing a cationic dye.
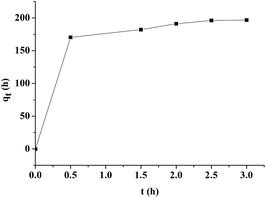 |
| | Fig. 6 Adsorption capacity of pfa@Fe2O3 (10 mg) to MB (200 mg l−1, 10 ml) during 3 h, T = 298 K. | |
 |
| | Fig. 7 Adsorption capacity of pfa@Fe2O3 (10 mg) at different concentrations of MB (10 ml), T = 298 K. | |
In order to further investigate the characteristics of the adsorption process, the pseudo second-order kinetic model was used to fit the experimental data obtained from the batch experiments as follows:
where,
qt and
qe were the concentrations of MB at time
t (min) and equilibrium,
k2 was the pseudo second-order reaction rate constant.
Fig. 8 and
Table 1 display kinetic curves for the adsorption of MB 200 mg l
−1 by the pfa@Fe
2O
3 (10 mg) at 299 K using the pseudo second-order kinetic model. An equation was used as follows:
R2 = 0.99777. The data in
Fig. 8 showed that the MB degradation curve was well fitted by the pseudo second-order kinetics with high values of the regression coefficients. As seen in the above equation,
qe = 199 mg g
−1,
k2 = 8.69 × 10
−6 mg g
−1 h
−1. These results indicated that the pfa@Fe
2O
3 showed excellent removal capacity for the MB in the solution.
 |
| | Fig. 8 The pseudo second order kinetics of pfa@Fe2O3 adsorbing MB. | |
Table 1 The adsorption kinetics data at different times
| Time/h |
qt/mg g−1 |
t/qt/h g mg−1 |
| 0 |
0 |
0 |
| 0.5 |
170.4 |
0.00294 |
| 1.5 |
182.2 |
0.00823 |
| 2 |
191.2 |
0.01046 |
| 2.5 |
196.2 |
0.01274 |
| 3 |
196.8 |
0.01524 |
MB@pfa@Fe2O3 as nanosorbcats for water oxidation
Water oxidation catalyzed by nanoparticles was carried out at room temperature under irradiation with red LED light (10 W) and blue LED light (10 W), respectively. For comparison, the photocatalytic activity of MB@pfa@Fe2O3, pfa@Fe2O3 and MB@recycled pfa@Fe2O3 were investigated under identical conditions. During the experimental process, we found that there was almost no evolution of O2 in pfa@Fe2O3, indicating that MB as photosensensitizer was an essential condition for photocatalytic water oxidation. It was interesting that both MB@pfa@Fe2O3 and MB@recycled pfa@Fe2O3 show a similar activity of water oxidation, which prove the better adsorption reusability of pfa@Fe2O3 from the others under identical conditions (Fig. S3†). As shown in Table 2 and Fig. S1,† the MB@pfa@Fe2O3/H2O system showed much higher activity with TON of 2.17 and TOF of 2.79 h−1, while the TON and TOF values for the pfa@Fe2O3/Ru(bpy)3Cl2/Na2S2O8 system were 0.87 and 1.30 h−1, respectively. These results clearly demonstrated that the catalyst created by the MB@pfa@Fe2O3 was more active than the traditional oxidation system (catalyst/RuII(bpy)3Cl2/Na2S2O8). There are two main reasons for this: firstly, the light absorption ability for methylene blue is better than Ru(bpy)3Cl2, thus the MB@pfa@Fe2O3/H2O system can use light energy more efficiently than the catalyst/Ru(bpy)3Cl2 system. Secondly, catalysts and photosensitizers were combined in the MB@pfa@Fe2O3/H2O system, so the light induced intramolecular charge transfer between the catalysts and photosensitizers in the MB@pfa@Fe2O3/H2O system more efficiently than in the catalyst/Ru(bpy)3Cl2 system. In comparison, the TON and TOF from the MB@pfa@Fe2O3 irradiated by the blue LED light were only 0.99 and 0.99 h−1, respectively (Fig. S2,† Table 2). It can therefore be concluded that the MB@pfa@Fe2O3/H2O system exerted higher activity when irradiated by the red LED light due to the maximum absorption capacity for the MB, which was at 664 nm. Furthermore, the MB@pfa@Fe2O3 also exerted high activity for water oxidation in aqueous solution conditions (Fig. 9), which indicated that the MB@pfa@Fe2O3/H2O could be used as a promising dioxygen generator or oxidant in hypoxic conditions using water as oxygen source. Moreover, the control experiments suggested that α-Fe2O3 couldn’t be LED-light driven catalysts for water oxidation. Hence, the pfa@Fe2O3 had higher activity for water oxidation than α-Fe2O3.
Table 2 Water oxidation activity of MB@pfa@Fe2O3 and pfa@Fe2O3/RuII(bpy)32+
| Catalysts |
TON |
TOF/h−1 |
| Irradiated with red LED light. Irradiated with blue LED light. TON = nO2 (μmol)/nFe (μmol), TOF = TON/time (h). |
| MB@pfa@Fe2O3a |
2.17 |
2.79 |
| pfa@Fe2O3/RuII(bpy)32+b |
0.87 |
1.30 |
| MB@pfa@Fe2O3b |
0.99 |
0.99 |
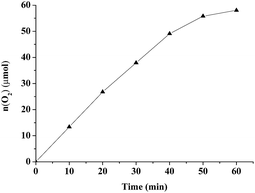 |
| | Fig. 9 Water oxidation activity of MB@pfa@Fe2O3 (10 mg) in aqueous solution at 298 K under irradiation of red LED light (10 W). | |
Adsorption reusability and possible mechanism
The recyclability and reusability of an adsorbent are very important for its practical applications. In this study, it was easy to recycle and reuse the pfa@Fe2O3. The regeneration of pfa@Fe2O3 after MB adsorption was conducted by flushing 3 times with ethanol and then drying in an oven. Fig. 10 shows the performance of the recycled pfa@Fe2O3. After three times of MB adsorption, the adsorption capacity was approximately 92% of the initial adsorption capacity. FT-IR spectra from the pfa@Fe2O3, MB@pfa@Fe2O3 (the first cycle) were also performed and obvious shifts were observed at 1596 and 1385 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (and C
N (and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) after adsorption by the pfa@Fe2O3, and also for C–N bonds in the heterocycle of MB and to 1436 cm−1 for CH2 deformation vibration in the benzene ring (Fig. 1c) which corresponded to the attachment of the MB on the surface of the pfa@Fe2O3 by π–π stacking interaction between the aromatic backbone of the MB and rhombic skeleton of pfa@Fe2O3, since the MB is an ideally planar molecule. Besides, the Ar–N deformation vibration and stretching vibrations for C
C) after adsorption by the pfa@Fe2O3, and also for C–N bonds in the heterocycle of MB and to 1436 cm−1 for CH2 deformation vibration in the benzene ring (Fig. 1c) which corresponded to the attachment of the MB on the surface of the pfa@Fe2O3 by π–π stacking interaction between the aromatic backbone of the MB and rhombic skeleton of pfa@Fe2O3, since the MB is an ideally planar molecule. Besides, the Ar–N deformation vibration and stretching vibrations for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and C–S were broadened with significant decreases in intensity, and they shifted to 1120 cm−1 and 1103 cm−1, respectively, suggesting that the sulfur atom from the C–S group could be used as hydrogen-bonding acceptor to form intramolecular hydrogen bonds with the H–Npyrrole of pfa@Fe2O3, since the MB is a cationic dye which can be adsorbed easily by electrostatic forces on the negatively charged surfaces.
S and C–S were broadened with significant decreases in intensity, and they shifted to 1120 cm−1 and 1103 cm−1, respectively, suggesting that the sulfur atom from the C–S group could be used as hydrogen-bonding acceptor to form intramolecular hydrogen bonds with the H–Npyrrole of pfa@Fe2O3, since the MB is a cationic dye which can be adsorbed easily by electrostatic forces on the negatively charged surfaces.
 |
| | Fig. 10 Adsorption reusability of pfa@Fe2O3 (10 mg) in MB solution (200 mg l−1, 10 ml) at 298 K. | |
Photocatalytic reusability
It is important for a photocatalyst to be stable and reusable for water oxidation. To investigate the stability of the MB@pfa@Fe2O3, recycling of the MB@pfa@Fe2O3 was conducted by flushing 3 times with water then drying in an oven. The recycled MB@pfa@Fe2O3 nanoparticles were then reused as photocatalyst. The TON and TOF were maintained as approximately 2 (Fig. 11). Furthermore, EPR data demonstrated that there was no significant change between the initial MB@pfa@Fe2O3 and recycled MB@pfa@Fe2O3, proving the stability of the MB@pfa@Fe2O3.
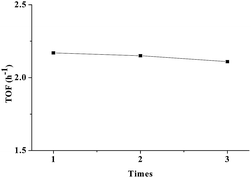 |
| | Fig. 11 Photocatalytic reusability of MB@pfa@Fe2O3 (10 mg) in water at 298 K and irradiation with red LED light (10 W). | |
Conclusions
The pyrro-2-methanic acid modified Fe2O3 nano-rods (pfa@Fe2O3) were successfully synthesized and characterized. It was found that the MB could be absorbed onto the pfa@Fe2O3 through hydrogen bonding and π–π stacking interaction between the aromatic backbone of the MB and rhombic skeleton of the pfa@Fe2O3. Both the absorption capacity and absorption rate were larger than those of the α-Fe2O3. The pfa@Fe2O3 could also be used as efficient reusable adsorbents for methylene blue (MB). In particular, the pfa@Fe2O3 and MB were combined into MB@pfa@Fe2O3 and this was an efficient recyclable nanosorbcat for LED-light driven dioxygen generation using water as oxygen source. Our results demonstrated that the conversion of efficient nanoadsorbents and valuable recyclable nanosorbcats can be an economic path for the treatment of colored waste water.
Acknowledgements
Financial support of the National Science Foundation of China (21271090, 21571085), the Youth Science Foundation of Jiangsu Province (BK20130485) and the Highly Qualified Professional Initial Funding of Jiangsu University (12JDG052) is acknowledged.
Notes and references
- H. S. Rai, M. S. Bhattacharyya, J. Singh, T. K. Bansal, P. Vats and U. C. Banerjee, Removal of dyes from the effluent of textile and dyestuff manufacturing industry: A review of emerging techniques with reference to biological treatment, Crit. Rev. Environ. Sci. Technol., 2005, 35, 219 CrossRef CAS.
- V. K. Gupta and Suhas, Application of low-cost adsorbents for dye removal, J. Environ. Manage., 2009, 90, 2313 CrossRef CAS PubMed.
- T. Yao, S. Guo, C. F. Zeng, C. Q. Wang and L. X. Zhang, Investigation on efficient adsorption of cationic dyes on porous magnetic polyacrylamide microspheres, J. Hazard. Mater., 2015, 292, 90 CrossRef CAS PubMed.
- S. Eftekhari, A. Habibi-Yangjeh and S. Sohrabnezhad, Application of AlMCM-41 for competitive adsorption of methylene blue and rhodamine B: Thermodynamic and kinetic studies, J. Hazard. Mater., 2010, 178, 349 CrossRef CAS PubMed.
- C. H. Huang, K. P. Chang, H. D. Ou, Y. C. Chiang, E.-E. Chang and C. F. Wang, Characterization and application of Ti-containing mesoporous silica for dye removal with synergistic effect of coupled adsorption and photocatalytic oxidation, J. Hazard. Mater., 2011, 186, 1174 CrossRef CAS PubMed.
- C. T. Frijters, R. H. Vos, G. Scheffer and R. Mulder, Decolorizing and detoxifying textile wastewater, containing both soluble and insoluble dyes, in a full scale combined anaerobic/aerobic system, Water Res., 2006, 40, 1249 CrossRef CAS PubMed.
- L. Wang, J. P. Zhang and A. Q. Wang, Removal of methylene blue from aqueous solution using chitosan-g-poly(acrylicacid)/montmorillonite superadsorbent nanocomposite, Colloids Surf., A, 2008, 322, 47 CrossRef CAS.
- Y. M. Zhou, S. Y. Fu, H. Liu, S. P. Yang and H. Y. Zhan, Removal of methylene blue dyes from wastewater using cellulose-based superadsorbent hydrogels, Polym. Eng. Sci., 2011, 51, 2417 CAS.
- L. Yao, L. Z. Zhang, R. Wang, S. R. Chou and Z. L. Dong, A new integrated approach for dye removal from wastewater by polyoxometalates functionalized membranes, J. Hazard. Mater., 2016, 301, 462 CrossRef CAS PubMed.
- Y. Liu, L. Yu, Y. Hu, C. Guo, F. Zhang and X. W. Lou, A magnetically separable photocatalyst based on nest-like γ-Fe2O3/ZnO double-shelled hollow structures with enhanced photocatalytic activity, Nanoscale, 2012, 4, 183 RSC.
- N. Anand, K. H. P. Reddy, T. Satyanarayana, K. S. R. Rao and D. R. Burri, A magnetically recoverable γ-Fe2O3 nanocatalyst for the synthesis of 2-phenylquinazolines under solvent-free conditions, Catal. Sci. Technol., 2012, 2, 570 CAS.
- Y. Fan, H. J. Liu, Y. Zhang and Y. Chen, Adsorption of anionic MO or cationic MB from MO/MB mixture using polyacrylonitrile fiber hydrothermally treated with hyperbranched polyethylenimine, J. Hazard. Mater., 2015, 283, 321 CrossRef CAS PubMed.
- A. X. Yan, S. Yao, Y. G. Li, Z. M. Zhang, Y. Lu, W. L. Chen and E. B. Wang, Incorporating polyoxometalates into a porous MOF greatly improves its selective adsorption of cationic dyes, Chem.–Eur. J., 2014, 20, 6927 CrossRef CAS PubMed.
- N. N. Nassar and A. Ringsred, Rapid adsorption of methylene blue from aqueous solutions by goethite nanoadsorbents, Environ. Eng. Sci., 2012, 29, 790 CrossRef CAS.
- S. Singh, K. C. Barick and D. Bahadur, Functional oxide nanomaterials and nanocomposites for the removal of heavy metals and dyes, Nanomater. Nanotechnol., 2013, 3, 1 CrossRef.
- C. J. Jones, S. Chattopadhyay, N. I. Gonzalez-Pech, C. Avendano, N. Hwang, S. Soo Lee, M. J. Cho, A. Ozarowski, A. Prakash, J. T. Mayo, C. Yavuz and V. L. Colvin, A novel, reactive green iron sulfide (sulfide green rust) formed on iron oxide nanocrystals, Chem. Mater., 2015, 27, 700 CrossRef CAS.
- L. W. Hou, L. G. Wang, S. Royer and H. Zhang, Ultrasound-assisted heterogeneous Fenton-likedegradation
of tetracycline over a magnetite catalyst, J. Hazard. Mater., 2016, 302, 458 CrossRef CAS PubMed.
- A. El-Qanni, N. N. Nassar, G. Vitale and A. Hassan, Maghemite nanosorbcats for methylene blue adsorption and subsequent catalytic thermo-oxidative decomposition: Computational modeling and thermodynamics studies, J. Colloid Interface Sci., 2016, 461, 396 CrossRef CAS PubMed.
- W. C. Ellis, N. D. McDaniel, S. Bernhard and T. J. Collins, Fast water oxidation using iron, J. Am. Chem. Soc., 2010, 132, 10990 CrossRef CAS PubMed.
- J. L. Fillol, Z. Codola, I. Garcia-Bosch, L. Gomez, J. J. Pla and M. Costas, Efficient water oxidation catalysts based on readily available iron coordination complexes, Nat. Chem., 2011, 3, 807 CrossRef CAS PubMed.
- C. Panda, J. Debgupta, D. D. Díaz, K. K. Singh, S. S. Gupta and B. B. Dhar, Homogeneous photochemical water oxidation by Biuret-Modified Fe-TAML: Evidence of FeV(O) intermediate, J. Am. Chem. Soc., 2014, 136, 12273 CrossRef CAS PubMed.
- J. A. Bau, E. J. Luber and J. M. Buriak, Oxygen evolution catalyzed by nickel–iron oxide nanocrystals with a nonequilibrium phase, ACS Appl. Mater. Interfaces, 2015, 7, 19755 CAS.
- R. D. L. Smith, M. S. Prévot, R. D. Fagan, S. Trudel and C. P. Berlinguette, Water oxidation catalysis: Electrocatalytic response to metal stoichiometry in amorphous metal oxide films containing iron, cobalt, and nickel, J. Am. Chem. Soc., 2013, 135, 11580 CrossRef CAS PubMed.
- J. J. Xue, Q. Y. Chen, X. L. Xu, S. J. He, P. X. Wu, L. L. Qu and C. Y. Zhu, Inorg. Chem. Commun., 2014, 47, 168 CrossRef CAS.
- L. Xu, J. X. Xia, L. G. Wang, J. Qian, H. M. Li, K. Wang, K. Y. Sun and M. Q. He, α-Fe2O3 cubes with high visible-light-activated photoelectrochemical activity towards glucose: Hydrothermal synthesis assisted by a hydrophobic ionic liquid, Chem.–Eur. J., 2014, 20, 2244 CrossRef CAS PubMed.
- Q. Zhang, D. G. John and R. G. Christian, C–H oxidation by H2O2 and O2 catalyzed by a non-heme iron complex with a sterically encumbered tetradentate N-donor ligand, Inorg. Chem., 2013, 52, 13546 CrossRef CAS PubMed.
- S. Y. Zhu, S. Fang, M. X. Huo, Y. Yu, Y. Chen, X. Yang, Z. Geng, Y. Wang, D. J. Bian and H. L. Huo, A novel conversion of the groundwater treatment sludge to magnetic particles for the adsorption of methylene blue, J. Hazard. Mater., 2015, 292, 173 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16049b |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (and C
N (and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) after adsorption by the pfa@Fe2O3, and also for C–N bonds in the heterocycle of MB and to 1436 cm−1 for CH2 deformation vibration in the benzene ring (Fig. 1c) which corresponded to the attachment of the MB on the surface of the pfa@Fe2O3 by π–π stacking interaction between the aromatic backbone of the MB and rhombic skeleton of pfa@Fe2O3, since the MB is an ideally planar molecule. Besides, the Ar–N deformation vibration and stretching vibrations for C
C) after adsorption by the pfa@Fe2O3, and also for C–N bonds in the heterocycle of MB and to 1436 cm−1 for CH2 deformation vibration in the benzene ring (Fig. 1c) which corresponded to the attachment of the MB on the surface of the pfa@Fe2O3 by π–π stacking interaction between the aromatic backbone of the MB and rhombic skeleton of pfa@Fe2O3, since the MB is an ideally planar molecule. Besides, the Ar–N deformation vibration and stretching vibrations for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and C–S were broadened with significant decreases in intensity, and they shifted to 1120 cm−1 and 1103 cm−1, respectively, suggesting that the sulfur atom from the C–S group could be used as hydrogen-bonding acceptor to form intramolecular hydrogen bonds with the H–Npyrrole of pfa@Fe2O3, since the MB is a cationic dye which can be adsorbed easily by electrostatic forces on the negatively charged surfaces.
S and C–S were broadened with significant decreases in intensity, and they shifted to 1120 cm−1 and 1103 cm−1, respectively, suggesting that the sulfur atom from the C–S group could be used as hydrogen-bonding acceptor to form intramolecular hydrogen bonds with the H–Npyrrole of pfa@Fe2O3, since the MB is a cationic dye which can be adsorbed easily by electrostatic forces on the negatively charged surfaces.









