Reflux precipitation polymerization: a new synthetic insight in molecular imprinting at high temperature†
Abstract
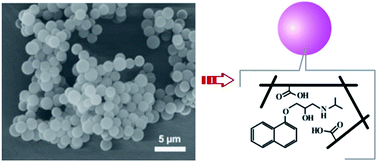
The synthesis of uniform molecularly imprinted polymer (MIP) microspheres (MSs) using distillation precipitation polymerization (DPP) at high temperature has attracted great interest in the field of molecular imprinting. However, there are still some shortcomings in this method. In this work, to create uniform MIP MSs in a short time and to demonstrate the effects of high temperature on imprinting performance, a new precipitation polymerization method (reflux precipitation polymerization, RPP) was used for the first time to fabricate MIP MSs in this study. The SEM images of the polymeric MSs indicate the presence of template molecules could improve the particle morphology and size uniformity. The specific molecular recognition of the monodispersed MIP MSs was confirmed by fluorescence measurement and HPLC-UV analysis. The binding behavior of the MIP MSs was simulated using the heterogeneous Freundlich isotherm, which shows that the MIP MSs produced by the RPP possess compatible selectivity in comparison with those produced by traditional PP method. It is noted that, for the first time, we demonstrated that molecular imprinting at high temperature was only successful when electrostatic interactions played important roles in the imprinting process.


 Please wait while we load your content...
Please wait while we load your content...