DOI:
10.1039/C6RA15983D
(Paper)
RSC Adv., 2016,
6, 94429-94436
Antibacterial and hemostatic composite gauze of N,O-carboxymethyl chitosan/oxidized regenerated cellulose†
Received
20th June 2016
, Accepted 19th September 2016
First published on 22nd September 2016
Abstract
Viscose gauze was oxidized with NO2/CCl4 to prepare Oxidized Regenerated Cellulose (ORC). Then, ORC was modified by the water-soluble chitosan derivative N,O-carboxymethyl chitosan (N,O-CS). This was synthesized via the reaction of chitosan with chloroacetic acid as the etherification agent in the presence of alkaline. It was prepared by introducing carboxymethyl groups onto the N and O-positions of the chitosan and the substituting degree (DS) of N,O-CS reached 1.68. Composite gauzes with a 2–8 w/v% N,O-CS content were prepared in water solution. Composite gauzes could still maintain their original morphological form and have excellent water-solubility. The formation of an amide bond between the carboxyl group of ORC and the amino group of N,O-CS was confirmed by FT-IR and elemental analysis. To study the influence of carboxymethyl groups on chitosan, the thermal stability and crystallinity of N,O-CS were tested by XRD and TG. Based on SEM images, N,O-CS tended to be adsorbed on the surface of ORC fiber. The antibacterial performance of N,O-CS/ORC gauzes was enhanced with the increases of N,O-CS content. Moreover, N,O-CS/ORC gauzes showed excellent bactericidal activity against both Gram-positive and Gram-negative bacteria. The hemostatic evaluation indicated that the N,O-CS/ORC composite gauzes in rabbit livers had dramatic hemostatic efficacy. The prepared composite gauzes were anticipated to be an optimal material in preventing post-operative adhesion.
Introduction
Oxidized regenerated cellulose (ORC) could be obtained by partial oxidation of the primary hydroxyl groups.1 This is generally used as an absorbable hemostatic agent in the vast majority of surgeries. In order to stop bleeding during surgical procedures effectively, ORC containing 16–24% carboxylic acid content is commercially available in the form of a powder or sterilized knitted fabric.2 Surgical trauma, hemorrhage, inflammation and pathogen contamination may be the main causes of adhesions. Based on the formation mechanism of adhesion and clinical experience, excellent hemostatic and antibacterial properties are required to prevent postoperative adhesion.3 However, with ORC it is difficult to ensure that all the blood is removed from the surgical field4 and ORC is mainly used to control low pressure where routine methods are not suitable or ineffective in preventing tissue adhesion in the presence of blood or body fluid.5,6 The relatively slow hemostatic rates as well as excessive acidic surface of ORC limit its wide applications in reducing adhesion.7,8
Chitosan (CS), a rare positive and alkaline polysaccharide, is well known for its extraordinary biological characteristics – it is nontoxic, nonantigenic, biodegradable, biocompatible, antibacterial, and hemostatic.9–13 However, its poor solubility in physiological solvents severely limits its further applications in the biomedical field.14 To overcome the above difficulty, several studies have successfully prepared water-soluble chitosan derivatives by chemical modification (the introduction of carboxymethyl groups on chitosan chains).15 Among the derivatives of chitosan, N,O-carboxymethyl chitosan (N,O-CS) has shown promise as an anti-adhesion agent, and can be processed into various forms, including solution, gel, or film. Numerous studies have confirmed that N,O-CS markedly diminishes postoperative adhesion in pericardial foreign body models and in abdominal cecal abrasion models.16 In addition, the N,O-CS product is efficacious in reducing adhesion formation in cardiac surgery. And N,O-CS products in the above forms are biodegradable within 5 days.17,18
In recent years, a few studies have considered C6-ORC gauze as a biomedical hemostatic material. They were coated by chitosan19 and functionalized multiwalled carbon nanotubes.20 However, no related report involved C6-ORC gauze coated by N,O-CS as postoperative adhesion materials. Compared with previous research,17 the N,O-CS/ORC composite gauzes didn't need to be further neutralized with NaOH/C2H5OH solution and could still be dissolved in aqueous solution. In this study, the main objective was to prepare a water-soluble gauze with an effective hemostatic and antibacterial material to improve the anti-adhesion property. Therefore, N,O-CS/ORC composite gauze was more suitably used for preventing postoperative adhesion in surgery. Briefly, an ORC coated N,O-CS C6-ORC gauze composite was prepared in a nitrogen dioxide (NO2)/carbon tetrachloride (CCl4) oxidation system. N,O-CS was synthesized from chitosan and chloroacetic acid with an appropriate concentration of alkaline. To improve the covalent bond between N,O-CS and ORC, 1-ethyl-3-(3-dimethylamino-propyl)-carbodiimide (EDC), N-hydroxyl-succinimide (NHS) and glycine as cross linking bridges were used to fabricate a novel N,O-CS/ORC composite gauze. And N,O-CS/ORC gauzes with different contents of N,O-CS were prepared. Then, the structures of N,O-CS and N,O-CS/ORC gauzes were characterized. Finally, the antibacterial and hemostatic properties of the composite gauzes were investigated. It is expected that this study could contribute to the design of effective postoperative anti-adhesion barrier material for future applications.
Experimental
Materials
CS (82.5% deacetylation degree (DD)) was obtained from Zhejiang Golden-Shell Biological Co., Ltd., China. Monochloroacetic acid and isopropyl alcohol were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Viscose filament yarn made of regenerated cellulose was obtained from Xinxiang city, Henan province, China. Nitrogen dioxide (AR, 99.99%, w/w) was purchased from Summit Specialty Gases Co., Ltd., Tianjin City, China. Carbon tetrachloride (AR, 99.5%) was purchased from Shuang Shuang Chemical Co., Ltd., Yantai City, China. 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and N-hydroxyl-succinimide (NHS) were purchased from J&K Chemical Co., Ltd. Glycine was supplied by Sinopharm Chemical Reagent Co., Ltd., China. As a control Surgicel® absorbable hemostat (commercial oxidized regenerated cellulose) was purchased from Johnson & Johnson Medical Limited. All reagents were used without further purification. Healthy rabbits were supplied by the animal experiment center of the second affiliated hospital of Harbin medical university (Harbin, Heilongjiang Province, China). The protocol was approved by the ethics committee of the Harbin Medical University. All animals were handled according to the Chinese National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Preparation of ORC
Prior to oxidation, regenerated cellulose filaments were oxidized using the method described in our previous works.19,21 Briefly, NO2 was dissolved in CCl4 to prepare the 20 wt% NO2/CCl4 oxidant, followed by the addition of regenerated cellulose into the flask, which contained the mentioned oxidant in a proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42.6 (g mL−1) (fiber
42.6 (g mL−1) (fiber![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxidant). Stirred constantly, the reaction was kept at a temperature of 19.5 °C and the oxidation duration was 40 h (shown in Fig. 1a). After the reaction, the product was washed with an aqueous solution containing 50% (v/v) isopropyl alcohol, and then the product was washed twice or more with 100% isopropyl alcohol. Finally, ORC was freeze-dried at −60 °C in vacuum for 24 h.
oxidant). Stirred constantly, the reaction was kept at a temperature of 19.5 °C and the oxidation duration was 40 h (shown in Fig. 1a). After the reaction, the product was washed with an aqueous solution containing 50% (v/v) isopropyl alcohol, and then the product was washed twice or more with 100% isopropyl alcohol. Finally, ORC was freeze-dried at −60 °C in vacuum for 24 h.
 |
| | Fig. 1 The possible oxidation reaction scheme for the preparation of ORC (a). Schematic illustration for the synthesis of N,O-carboxymethyl chitosan (N,O-CS) (b). | |
Synthesis of N,O-carboxymethyl chitosan (N,O-CS)
N,O-CS was synthesized as described in the literature with some modifications.22–24 Briefly, 10 g of chitosan was suspended in 100 mL of isopropyl alcohol and the resulting slurry was stirred in a flask at room temperature. 25 mL of 10 N aqueous NaOH solution was divided into five equal portions and added to the stirred slurry over a period of 25 min. The alkaline slurry was stirred for an additional 30 min. Then monochloroacetic acid (20 g) was added dropwise in five equal portions at 5 min intervals. Heat was then applied to bring the reaction mixture to a temperature of 60 °C and then stirred for 3 h. Subsequently, the reaction mixture was filtered and the residue solid product (N,O-CS) was thoroughly rinsed three times with 80% v/v ethanol solution and several times with 100% ethanol. The dry product was obtained by vacuum-drying. The chemical reaction is schematized in Fig. 1b.
DS measurement
The DS of N,O-CS was measured using potentiometric titration method. N,O-CS (0.2 g) was dissolved in a 20 mL 0.1 mol L−1 HCl standard solution and titrated by a 0.1 mol L−1 NaOH standard solution. The potentiometric titration curve is shown in Fig. 2. The second order micro commercial law was used to get the sudden-change point to calculate the DS of N,O-CS according to the following formulas:| |
 | (1) |
| |
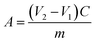 | (2) |
| |
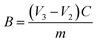 | (3) |
| |
 | (4) |
where V1 is the potentiometric titration end-point of excessive HCl (mL); V2 is the potentiometric titration end-point of –COOH (mL); V3 is the potentiometric titration end-point of –NH+ (mL); C is the molar concentration of NaOH solution (mol L−1); m is the weight of N,O-CS (g); DD is the degree of deacetylation of chitosan (82.5%); DSN is the DS on NH2; DS0 is the DS on OH.
 |
| | Fig. 2 Potentiometric titration curve of N,O-CS. | |
Preparation of N,O-CS/ORC gauze
The optimal group of N,O-CS was used to prepare composite gauzes with ORC, as shown in Fig. 3. N,O-CS solution was prepared through the method of being dissolved in distilled water to 2–8 w/v%. Then, EDC, NHS and glycine were added to the N,O-CS solution and stirred using a magnetic stirrer for 10 min.
 |
| | Fig. 3 Diagram of the preparation of N,O-CS/ORC composites. | |
Finally, the ORC was immersed in N,O-CS solution and stirred at room temperature for 6 h. After the above treatment, the ORC dipped into the N,O-CS was washed by pressure filtration with an 80% v/v ethanol solution three times and with 100% ethanol several times. Then, the modified ORC was freeze-dried at a low temperature (223 K) in vacuum for 24 h. For comparison, a sample of ORC was also prepared without the addition of N,O-CS.
Characterization of N,O-CS and N,O-CS/ORC gauze
Fourier transformed infrared spectra (FT-IR) and proton nuclear magnetic resonance spectroscopy (1H-NMR) were used to confirm the substitutions of carboxymethyl groups on the amino and primary hydroxyl sites of the modified chitosan (N,O-CS). The sample for FT-IR was scanned from 400 to 4000 cm−1. 1H-NMR spectra were performed at room temperature with a Varian 400 spectrometer.
Carboxyl group content determination
Carboxyl content was measured according to United States Pharmacopoeia. Firstly, 2% calcium acetate solution and 0.1 M NaOH standard solution were prepared. Then, 0.5 g ORC was cut into short fibers and soaked with 50 mL 2% calcium acetate solution for 15 h. Using phenolphthalein as an indicator, the above mixture was titrated with a 0.1 M NaOH standard solution. And the consumed volume of the NaOH standard solution was corrected for a blank. The carboxyl content could be calculated from the following equation:19| |
 | (7) |
where N is the normality of 0.1 M NaOH solution; V is the consumed volume of NaOH which is corrected for the blank; MWCOOH presents the molecular weight of the carboxyl group and m is the weight of the sample.
Elemental analysis
The carbon (C) and nitrogen (N) contents of the composite gauzes and ORC samples were determined by elemental analysis using the CHN module of a PerkinElmer Series II 2400 (USA) CHNS/O Elemental Analyzer. The analysis was repeated three times. Each sample had a weight range of 1.800–3.200 mg.
X-ray diffraction
The crystallinity of N,O-CS, chitosan, and composite gauzes was measured by an X-ray diffraction method using an XRD-6000X diffractometer with Cu Kα X-radiation. The samples were recorded over a diffraction angle (2θ) that ranged from 10° to 60°.
Thermal analysis
Thermogravimetric analyses (TGA) for N,O-CS, chitosan and composite gauzes were recorded on a SDT Q600 TGA instrument. The samples weighing around 3 mg were heated from 50 to 500 °C at a heating rate of 10 °C min−1 and under an inert atmosphere of nitrogen.
Scanning electron microscopy (SEM) analysis
SEM was used to investigate the surface morphologies of composite gauzes and the connection between N,O-CS and ORC. The samples were sputter-coated with gold for better conductivity during imaging. Then the samples were observed with SEM (Hitachi, S-3400) at an acceleration voltage of 5 kV.
Antibacterial activity test
The GB/T 20944.3-2008 test method was applied to determine the antibacterial activity. Gram positive bacterium, Staphylococcus aureus (S. aureus)-ATCC 6538, and Gram negative bacterium, Escherichia coli (E. coli)-ATCC 8739, were used as the test organisms. Bacterial inocula were prepared to obtain a bacterial suspension at an exponential growth of 108 colony forming units (CFU) mL−1 in 5 mL nutrient broth (modified tryptone soya broth from Oxoids). A tryptone soya agar (from Oxoids) was used as the nutrient agar for the agar plates. Antibacterial tests were carried out on several batches of ORC gauzes coated with N,O-CS. The untreated cotton gauze was used as a control sample. The circular fabric swatches were cut to 4 cm in diameter and then sterilized under Co60 irradiation. Each fabric sample was placed in a flask. The antibacterial test was performed according to the AATCC 100-2004 test standard. A series of diluted solutions were prepared as 100, 101, 102, and 103 times with sterile distilled water. Then they were plated (3 of each) and incubated for 18–24 h at 37 °C. After incubation, the sample of dilution 102 was chosen to compare the treated and untreated samples. The plates corresponding to 100 and 101 times colonies were uncountable and that for 103 times had too few colonies for the untreated control sample. The reduction percentage of bacteria by the gauze specimen treatment was calculated through the following formula:24–26| | |
Reduction in CFU (%) = (Ct − Tt)/Ct × 100%
| (8) |
where Ct is the average number of bacterial colonies in untreated cotton gauze; Tt is the average number of bacterial colonies in ORC gauze treated with N,O-CS.
Hemostatic evaluation
The hemostatic behavior of the N,O-CS/ORC composite gauzes was estimated by covering gauze on the abraded liver of male New Zealand white rabbits (4 months old and around 3.5 kg). The neat ORC and N,O-CS/ORC gauzes were cut into pieces of the required size (2.0 cm × 2.0 cm) and sterilized by ultraviolet radiation for testing the hemostatic efficiency. Before undergoing an abdominal incision, the rabbits were fixed on the surgical cork board and anesthetized with an intraperitoneal injection of 3% pentobarbital sodium aqueous solution (30 mg kg−1). The neat ORC and its composite gauzes were applied to the liver wound immediately when the liver was pricked with a needle (the diameter is 2 mm, and the pricked depth is 3 mm). In this step, after three minutes for blood penetration in the samples and the clot formation on the surface of specimens was easily observed.
Results
Synthesis of N,O-carboxymethyl chitosan (N,O-CS)
N,O-CS, a derivative of chitosan, was synthesized by the introduction of carboxymethyl groups into the N-terminal and O-terminal of CS, as shown in Fig. 1. The determined substitution (DS) degree of N,O-CS was up to 1.68. The free amine group content after the carboxymethylation of chitosan was 17.03%. The carboxymethyl group was mainly introduced into OH at C6 and C3 with a small number of NH2 at C2 (DS0/DS > 50%). The goal of preparing N,O-CS with a high DS and a low DSN was realized and the antibacterial activity of the chitosan was not influenced seriously27 due to the fact that nucleophilic substitution reaction of NH2 at C2 could react not only with OH at C6, but also with OH at C3. The electronegativity of O was stronger than that of N. And the steric hindrance of OH at C6 is the smallest. Therefore, the sequence of substitution site was: OH-6 > OH-3 > NH2-2.28
Carboxyl content of composite gauzes
Oxidized regenerated cellulose was obtained by NO2/CCl4 selective oxidation of viscose gauze, leading to the formation of ORC which contains the carboxyl group. Fig. 4 shows that the carboxyl content decreased from 18.15% to 6.25% while the content of N,O-CS increased from 0 to 8%. This phenomenon might be caused by a chemical reaction between N,O-CS and the ORC gauze, with new chemical bonds emerging.
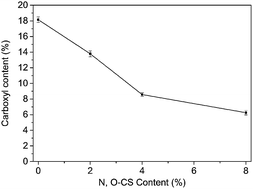 |
| | Fig. 4 The relationship between carboxyl content and different N,O-CS content. | |
Elemental analysis
Elemental analysis can be employed to analyze the compositions of the N,O-CS/ORC gauzes and ORC. The results are listed in Table 1, showing that the nitrogen proportion increased, ranging from 1.20 to 1.52%, with the increase of the N,O-CS content (2% to 8%). The results proved the formation of new chemical bonds (amide bonds) between N,O-CS and ORC gauze.
Table 1 The data of elemental analysis
| Sample |
C% |
H% |
N% |
| ORC |
39.72 ± 0.15 |
5.13 ± 0.13 |
— |
| 2% N,O-CS |
34.63 ± 0.28 |
4.91 ± 0.34 |
1.20 ± 0.15 |
| 4% N,O-CS |
34.52 ± 0.18 |
5.21 ± 0.20 |
1.40 ± 0.30 |
| 8% N,O-CS |
34.85 ± 0.33 |
5.24 ± 0.19 |
1.52 ± 0.28 |
FT-IR
As shown in Fig. 5a, chitosan showed its unique characteristic peaks at 3435, 1658, 1075 and 1030 cm−1, which were attributed to the stretch and vibration of the O–H, N–H, and amino groups (NH2 deformation), the second hydroxyl group (C–O) and the primary hydroxyl group (C–O–C), respectively.29 In contrast, the spectrum of N,O-CS showed characteristic peaks at 1606 and 1412 cm−1 corresponding to the carboxylic acid salt (–COO− asymmetric stretch) and the –COONa stretch, which showed the existence of carboxymethyl groups on N,O-CS. Furthermore, peaks at 1075 and 1030 cm−1 represented a secondary hydroxyl group (–CH–OH, C–O) and a primary hydroxyl group (–CH2–OH, C–O), respectively, because the secondary hydroxyl group was not affected by the modification of chitosan. The results showed that the peak intensity ratio (1030 cm−1/1070 cm−1) of N,O-CS decreased compared with that of CS,22 demonstrating the substitution of a carboxymethyl group for –CH2–OH at the C6 position of N,O-CS. Compared with the 1H-NMR spectrum of N,O-CS (Fig. S1, ESI†), we observed new signals at 2.6 ppm in the spectrum of N,O-CS. This corresponds to the carboxymethyl group grafted onto the NH2 at C2 and OH at C6, which was consistent with a previous research study.23
 |
| | Fig. 5 (a) FT-IR spectra of chitosan and N,O-CS, (b) FT-IR spectra of ORC and composite gauzes with different N,O-CS content. | |
A characteristic absorption band of the ORC clearly appeared at 1740 cm−1 due to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (–COOH group). Treated with N,O-CS, peaks at 1606 and 1410 cm−1 corresponding to the carboxylic acid salt (–COO− asymmetric stretch and –COONa symmetry stretch), showed the existence of a carboxymethyl group (Fig. 5b). The spectra of composite gauzes with different N,O-CS content are similar.
O (–COOH group). Treated with N,O-CS, peaks at 1606 and 1410 cm−1 corresponding to the carboxylic acid salt (–COO− asymmetric stretch and –COONa symmetry stretch), showed the existence of a carboxymethyl group (Fig. 5b). The spectra of composite gauzes with different N,O-CS content are similar.
And the absorption strength became strong with an increase of N,O-CS content. The absorption strength at 1740 cm−1 was weak, but still could be seen with an increase of N,O-CS content (shown in Fig. 5b), implying that an amide bond (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N bond) formed between –COOH groups in ORC and amino groups in N,O-CS. Compared with the spectrum of pure ORC, O–H and N–H stretching peaks at 3415 cm−1 in N,O-CS/ORC gauze became wider and shifted to a higher wave number, which showed the existence of the interaction between N,O-CS and ORC gauze.
C–N bond) formed between –COOH groups in ORC and amino groups in N,O-CS. Compared with the spectrum of pure ORC, O–H and N–H stretching peaks at 3415 cm−1 in N,O-CS/ORC gauze became wider and shifted to a higher wave number, which showed the existence of the interaction between N,O-CS and ORC gauze.
XRD
X-ray diffractions of N,O-CS, chitosan and composite gauzes are given in Fig. 6. It shows that the XRD profiles of chitosan exhibited a typical diffraction angle (2θ) around 20.1°, while N,O-CS exhibited a typical diffraction angle (2θ) around 20.5° and was almost amorphous. Because of the introduction of the carboxymethyl group, the distance between molecular chains was destroyed and the original crystal structure sharply increased.30
 |
| | Fig. 6 XRD of chitosan, N,O-CS, ORC, and N,O-CS/ORC composite gauze. | |
XRD diffraction peaks of ORC showed two weak typical diffraction angles (2θ) around 20.3° and 21.8°. The reason is that the crystallinity structure of ORC was destroyed during the oxidation reaction to give an almost amorphous structure. The peaks had an apparently lower diffraction angle in the composite gauze and the intensity of diffraction peaks weakened after the N,O-CS was coated, which demonstrated that an interaction occurred between the N,O-CS and ORC gauze.
TGA
TGA of chitosan, N,O-CS, ORC and N,O-CS/ORC composite gauze were carried out to evaluate their degradation profiles and thermal stability. TGA curves are shown in Fig. 7. In the thermogravimetry of chitosan, the TGA curve of the pyrolysis process could be divided into two weight loss stages, consistent with a slow pyrolysis process (50–100 °C) and a second pyrolysis (250–400 °C) stage. At the first stage, the mass loss was contributed to volatilization of water. The maximum rate of weight loss was observed in the second stage, where over 56% weight was pyrolyzed between 250 °C and 400 °C. In contrast, TGA curve of N,O-CS could also be divided into two stages, the thermal stability of N,O-CS was weaker than that of chitosan since the onset of thermal degradation of N,O-CS was about 225 °C and the maximum rate of weight loss of N,O-CS was only about 23.9% between 225 °C and 300 °C (ref. 24) due to the process of carboxymethylation together with the rupture of macromolecule chains of chitosan by alkali and a high temperature. Moreover, the above factors contributed to the decrease and even damage of the polymerization degree of chitosan. Therefore, chitosan not only had a better thermo stability than N,O-CS, but also a higher pyrolysis than N,O-CS.
 |
| | Fig. 7 TGA curves of chitosan, N,O-CS, ORC, and N,O-CS/ORC composite gauze. | |
The concentration of N,O-CS on the surface of ORC was estimated using TGA. As shown in Fig. 7, the maximum thermal degradation temperature of ORC before and after being coated with N,O-CS was about 200 °C. And the maximum rate of weight loss before and after N,O-CS coating of ORC decreased (56.97% and 48.74%) between 200 and 300 °C, which further demonstrated the formation of a chemical bond between N,O-CS and ORC consistent with the FT-IR and XRD data.
SEM
Composite gauzes with various N,O-CS contents were examined by SEM as presented in Fig. 8. The surface morphology of ORC fibers was rough (Fig. 8a). Meanwhile, coating happened on composite gauzes, and a layer of N,O-CS was absorbed on the surface of ORC fibers, implying that N,O-CS was connected with the ORC fibers during the modification. Also, it can be seen that some of the N,O-CS grains connected two neighbor ORC fibers to each other. This effect promises an increase in the antibacterial and hemostatic properties of ORC fibers (Fig. 8b and c).
 |
| | Fig. 8 SEM images of ORC and N,O-CS/ORC fibers. (a) ORC fibers. (b) N,O-CS/ORC fibers (5000×). (c) N,O-CS/ORC fibers (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×). 000×). | |
Characterization of water-soluble N,O-CS-coated ORC gauze
In second stage, after N,O-CS coating of the ORC gauze, the N,O-CS/ORC gauze was neutralized to gain the water-soluble sodium salts. Water soluble characteristics of N,O-CS-coated ORC composite gauze are given in Fig. 9.31 Saferstein et al. discovered that neutralizing oxidized cellulose cloth in aqueous solutions of sodium bicarbonate led to what was a partial gel, distorted from its original size, very weak, and with little integrity. The tensile strength of the cloth was too low to use as a hemostat in surgery. While in our experiment, comparing Fig. 9a and d, we could find that for N,O-CS coated ORC gauze, the composite gauze totally lost their original physical forms and generate a transparent gel quickly for 10 s after the gauzes are soaked with enough water (Fig. 9e and f). This is due to the N,O-CS coated ORC gauze because the sodium carboxylate (COONa) groups have been introduced into the materials, which are water-soluble. Thus, the N,O-CS/ORC composite gauze could be dissolved in an aqueous solution, while the gauze before the N,O-CS coating could not be dissolved into the aqueous solution with only a swelling behavior in aqueous solution (Fig. 9b and c).
 |
| | Fig. 9 The digital pictures of N,O-CS/ORC gauze before and after coating with N,O-CS dissolved in water. (a)–(c) Before coating. (d)–(f) After coating. | |
Antibacterial activity test
The antibacterial activity of ORC and N,O-CS/ORC gauzes was evaluated against Gram positive and negative bacteria, S. aureus and E. coli. The results are indicated in Table 2. The inhibitory rate against S. aureus and E. coli apparently increased with the increase of N,O-CS content. This implied that the strong antibacterial activity of N,O-CS compensated for the decreasing antibacterial property of ORC during the consumption of some –COOH groups. Most researchers reported that the antibacterial property of ORC depended on its pH. A lower pH gave a better antibacterial property of ORC. In addition, most literature25,26 reported that chitosan killed bacteria through damage to the cell membrane and this damage was caused by electrostatic interactions between chitosan protonated amino groups and phosphoryl groups of phospholipid components of the cell membranes. And the inhibitory rate against S. aureus was higher than that against E. coli with an identical N,O-CS content.
Table 2 The bactericidal properties of different N,O-CS/ORC gauzes
| Samples |
S. aureus (%) |
E. coli (%) |
| ORC |
>94.65 |
>92.89 |
| 2% N,O-CS/ORC |
>95.66 |
>93.57 |
| 4% N,O-CS/ORC |
>97.38 |
>95.25 |
| 8% N,O-CS/ORC |
>99.99 |
>97.68 |
| Surgical |
>99.99 |
>99.99 |
Hemostatic capability
There are several factors that affect the bleeding of the white rabbit liver, for example, the blood pressure and wound size of the liver. In order to reduce experimental error, three rabbits for each specimen were used. The results of hemostatic capability are shown in Fig. 10. The rabbit liver experiments indicated that both the neat ORC and the N,O-CS/ORC composite gauze could stop bleeding and decrease blood coagulation on the surface of the liver. The use of the N,O-CS/ORC composite gauze stopped bleeding in 90 seconds, while there was still bleeding points in ORC gauze (stopped bleeding in 4 minutes). The average blood uptake of neat ORC was 359.8% and that of N,O-CS/ORC was 128.1%. The reasons for this phenomenon are that, firstly, the unreacted –COOH groups in ORC had the capability to bind with Fe3+ in the blood fluid to form a brown gel. Secondly, the water-soluble –COONa groups would absorb water from the blood and make the gauze gel quickly and concentrate the clotting factors to carry out hemostasis. In this way, a rapid coagulation rate would give a smaller amount of blood loss and clot formation. As we all know, the clot will affect the efficacy of tissue anti-adhesion. This indicated that N,O-CS/ORC composite gauzes are beneficial for postoperative adhesion prevention.
 |
| | Fig. 10 Images of an injured site of rabbit liver (a), hemostatic evaluation of neat ORC (b) and N,O-CS/ORC composite gauze (c). | |
Conclusions
In this research, a novel technique was invented to graft ORC with a water-soluble N,O-CS via amide bonds. At first, N,O-CS was successfully prepared by modifying chitosan with chloroacetic acid in alkaline solution. Then, ORC gauze was coated with the above N,O-CS. The formation of an amide bond between the carboxyl group of ORC and the amino group of N,O-CS was confirmed by FT-IR and elemental analysis. Antibacterial properties of N,O-CS/ORC composite gauzes increased with increasing of N,O-CS content. The N,O-CS coated ORC gauze was water-soluble and was able to form a gel by absorbing blood and then sealing off the crevasses of the blood vessels to stop bleeding. This research indicates that the hemostatic performance of ORC can be improved by introducing a small proportion of N,O-CS. The notable properties of N,O-CS/ORC composite gauzes have promising future applications in reducing the formation of postoperative adhesions after surgery.
Acknowledgements
This work was financially supported by the State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University (No. LK1510).
Notes and references
- M. N. V. R. Kumar, React. Funct. Polym., 2000, 46, 1–27 CrossRef CAS.
- L. H. Zhu, V. Kumar and G. S. Banker, Int. J. Pharm., 2001, 223, 35–47 CrossRef CAS PubMed.
- H. Wang, M. Li, J. Hu, C. Wang, S. Xu and C. C. Han, Biomacromolecules, 2013, 14, 954–961 CrossRef CAS PubMed.
- Q. Xia, Z. Liu, C. Wang, Z. Zhang, S. Xu and C. C. Han, Biomacromolecules, 2015, 16, 3083–3092 CrossRef CAS PubMed.
- Y. C. Chung, Y. P. Su, C. C. Chen, G. Jia, H. L. Wang, J. C. G. Wu and J. G. Lin, Acta Pharmacol. Sin., 2004, 25, 932–936 CAS.
- E. Lih, S. H. Oh, Y. K. Joung, J. H. Lee and D. K. Han, Prog. Polym. Sci., 2015, 44, 28–61 CrossRef CAS.
- B. C. Ward, S. Kavalukas, J. Brugnano, A. Barbul and A. Panitch, J. Surg. Res., 2011, 169, e27–36 CrossRef CAS PubMed.
- G. M. Boland and R. J. Weigel, J. Surg. Res., 2006, 132, 3–12 CrossRef PubMed.
- A. M. Abdelgawad, S. M. Hudson and O. J. Rojas, Carbohydr. Polym., 2014, 100, 166–178 CrossRef CAS PubMed.
- R. A. A. Muzzarelli, P. Morganti, G. Morganti, P. Palombo, M. Palombo, G. Biagini, M. Mattioli Belmonte, F. Giantomassi, F. Orlandi and C. Muzzarelli, Carbohydr. Polym., 2007, 70, 274–284 CrossRef CAS.
- K. Sun, eXPRESS Polym. Lett., 2011, 5, 342–361 CrossRef CAS.
- G. Toskas, C. Cherif, R. D. Hund, E. Laourine, B. Mahltig, A. Fahmi, C. Heinemann and T. Hanke, Carbohydr. Polym., 2013, 94, 713–722 CrossRef CAS PubMed.
- F. Cheng, J. Gao, L. Wang and X. Y. Hu, J. Appl. Polym. Sci., 2015, 132, 42060 Search PubMed.
- M. G. McKee, J. M. Layman, M. P. Cashion and T. E. Long, Science, 2006, 311, 353–355 CrossRef CAS PubMed.
- A. J. Varma, S. V. Deshpande and J. F. Kennedy, Carbohydr. Polym., 2004, 55, 77–93 CrossRef CAS.
- T. J. Krause, G. A. Zazanis and R. D. McKinnon, Wound Repair Regen., 1996, 4, 53–57 CAS.
- J. Zhou, J. M. Lee, P. Jiang, S. Henderson and T. D. G. Lee, J. Thorac. Cardiovasc. Surg., 2010, 140, 801–806 CrossRef CAS PubMed.
- J. Zhou, R. S. Liwski, C. Elson and T. D. G. Lee, J. Thorac. Cardiovasc. Surg., 2008, 135, 777–783 CrossRef CAS PubMed.
- J. M. He, F. W. Wang, Y. D. Wu, Y. D. Huang and H. W. Zhang, Cellulose, 2011, 18, 1651–1659 CrossRef CAS.
- A. N. Chakoli, J. M. He, W. L. Cheng and Y. D. Huang, RSC Adv., 2014, 4, 52372–52378 RSC.
- W. L. Cheng, J. M. He, Y. D. Wu, C. Song, S. S. Xie, Y. D. Huang and B. Fu, Cellulose, 2013, 20, 2547–2558 CrossRef CAS.
- S. C. Chen, Y. C. Wu, F. L. Mi, Y. H. Lin, L. C. Yu and H. W. Sung, J. Controlled Release, 2004, 96, 285–300 CrossRef CAS PubMed.
- L. Li, N. Wang, X. Jin, R. Deng, S. H. Nie, L. Sun, Q. J. Wu, Y. Q. Wei and C. Y. Gong, Biomaterials, 2014, 35, 3903–3917 CrossRef CAS PubMed.
- X. Zheng, H. Zhang, Y. She and J. W. Pu, J. Appl. Polym. Sci., 2014, 131, 39851 Search PubMed.
- Y. D. Wu, J. M. He, F. W. Wang, W. L. Cheng, Y. D. Huang and B. Fu, Fibers Polym., 2014, 15, 504–509 CrossRef.
- J. M. He, Y. D. Wu, Y. D. Huang, F. W. Wang and F. Tang, Fibers Polym., 2012, 13, 576–581 CrossRef.
- I. Aranaz, M. Mengíbar, R. Harris, I. Paños, B. Miralles, N. Acosta, G. Galed and Á. Heras, Curr. Chem. Biol., 2009, 3, 203–230 CAS.
- H. K. Chun, J. W. Choi, J. C. Heung and S. C. Kyu, Polym. Bull., 1997, 38, 387–393 CrossRef.
- K. Mladenovska, R. S. Raicki, E. I. Janevik, T. Ristoski, M. J. Pavlova, Z. Kavrakovski, M. G. Dodov and K. Goracinova, Int. J. Pharm., 2007, 342, 124–136 CrossRef CAS PubMed.
- C. Zhang, Q. E. Ping, H. J. Zhang and J. Shen, Eur. Polym. J., 2003, 39, 1629–1634 CrossRef CAS.
- L. Saferstein, S. Wolf, L. Kamp, C. Linsky and D. Wiseman, US pat., 5134229, 1992.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR spectra of N,O-CS. See DOI: 10.1039/c6ra15983d |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42.6 (g mL−1) (fiber
42.6 (g mL−1) (fiber![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxidant). Stirred constantly, the reaction was kept at a temperature of 19.5 °C and the oxidation duration was 40 h (shown in Fig. 1a). After the reaction, the product was washed with an aqueous solution containing 50% (v/v) isopropyl alcohol, and then the product was washed twice or more with 100% isopropyl alcohol. Finally, ORC was freeze-dried at −60 °C in vacuum for 24 h.
oxidant). Stirred constantly, the reaction was kept at a temperature of 19.5 °C and the oxidation duration was 40 h (shown in Fig. 1a). After the reaction, the product was washed with an aqueous solution containing 50% (v/v) isopropyl alcohol, and then the product was washed twice or more with 100% isopropyl alcohol. Finally, ORC was freeze-dried at −60 °C in vacuum for 24 h.


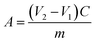
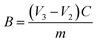



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (–COOH group). Treated with N,O-CS, peaks at 1606 and 1410 cm−1 corresponding to the carboxylic acid salt (–COO− asymmetric stretch and –COONa symmetry stretch), showed the existence of a carboxymethyl group (Fig. 5b). The spectra of composite gauzes with different N,O-CS content are similar.
O (–COOH group). Treated with N,O-CS, peaks at 1606 and 1410 cm−1 corresponding to the carboxylic acid salt (–COO− asymmetric stretch and –COONa symmetry stretch), showed the existence of a carboxymethyl group (Fig. 5b). The spectra of composite gauzes with different N,O-CS content are similar.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N bond) formed between –COOH groups in ORC and amino groups in N,O-CS. Compared with the spectrum of pure ORC, O–H and N–H stretching peaks at 3415 cm−1 in N,O-CS/ORC gauze became wider and shifted to a higher wave number, which showed the existence of the interaction between N,O-CS and ORC gauze.
C–N bond) formed between –COOH groups in ORC and amino groups in N,O-CS. Compared with the spectrum of pure ORC, O–H and N–H stretching peaks at 3415 cm−1 in N,O-CS/ORC gauze became wider and shifted to a higher wave number, which showed the existence of the interaction between N,O-CS and ORC gauze.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×).
000×).






